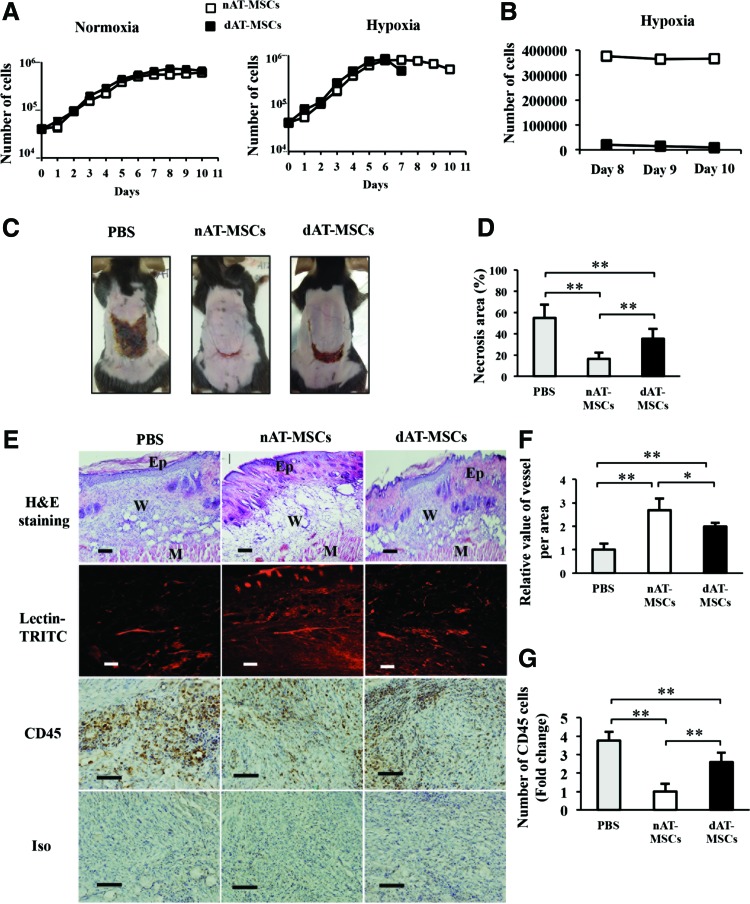
FIG. 2.
The ability to improve wound healing is impaired in dAT-MSCs in the mouse skin flap model. (A) The number of cells was counted every 24 h using Trypan Blue exclusion over 10 days under normoxic (20% O2) or hypoxic (5% O2) conditions. Abnormal cell adhesion was observed in dAT-MSCs under hypoxic conditions on day 7. The precise number of cells could not be counted because the cells adhered together too tightly. The average doubling time was 32.5 ± 2 h under normoxic conditions and 28.5 ± 2 h under hypoxic conditions (P < 0.01). (B) The number of dAT-MSCs was counted after abnormal cell adhesion and compared with the number of nAT-MSCs on days 8, 9, and 10. (C) Images of the necrotic areas of mice injected with PBS, nAT-MSCs, and dAT-MSCs were captured on day 7 after injection. (D) The percentage of the necrotic area was calculated based on the necrotic area per wound area in mice that were injected with PBS (n = 5), nAT-MSCs (n = 30), and dAT-MSCs (n = 30). (E) The embedded sections were examined by H&E staining for tissue structure, which revealed the epidermis, wound, and muscle in each wound site; BS-I Lectin-TRITC was injected into the tail vein to observe the vessel formation (red) with fluorescence intensity; and the CD45 immunohistochemical staining of inflammatory cells (brown). (F) The fluorescence intensity was measured using the ImageJ software program to evaluate the number of vessels in each area (n = 20). (G) The CD45-positive cells were counted and expressed relative to the number nAT-MSCs (n = 10). Data represent the average of three independent experiments (mean ± SD); *P < 0.05; **P < 0.01. Scale bars: 100 μm; BS-I, Bandeiraea simplicifolia-I; Ep, epidermis; M, muscle; W, wound. Color images available online at www.liebertpub.com/scd