Figure 3.
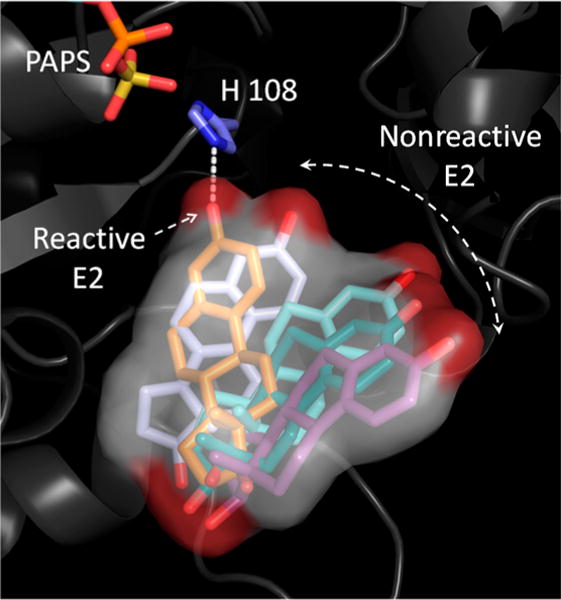
Wandering nucleophile. Snapshots from a GROMACS simulation reveal how E2 (a neutral-synergy substrate) wanders in and out of catalytic position when bound in the E·PAPS·E2 complex. E2 is estimated to spend approximately 2% of its time in a catalytically active position. Positive-synergy substrates are predicted to spend ~100% of the time in an active position, and this difference may explain the roughly 100-fold differences in kcat associated with positive- and neutral-synergy substrates. Similar behaviors were observed with two positive-synergy and two neutral-synergy acceptors: Acet and 2-Nap, respectively, and E2 and DHEA, respectively. Movies that present the predicted motions of these acceptors in the SULT1A1 active-site cavities are available as Supporting Information.
