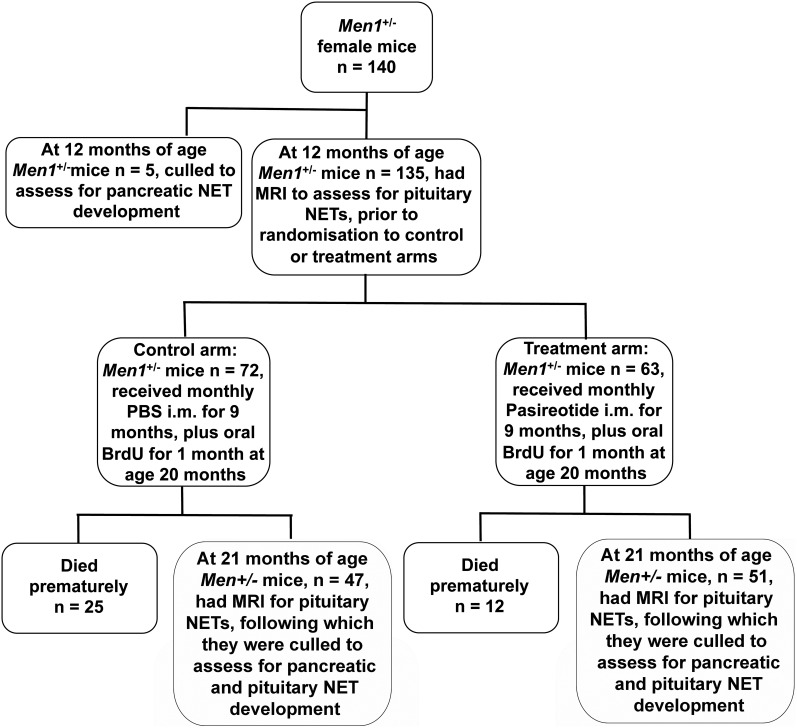
Figure 1.
Flowchart for the trial of pasireotide in the treatment of neuroendocrine tumors (NETs) of Men1+/− mice. Female Men1+/− mice (n = 140) were aged to 12 mo, and 5 Men1+/− mice (control nontreated 12-mo group) were culled to assess for the number of pancreatic NETs. The remaining 135 Men1+/− mice underwent MRI for assessment of pituitary NETs, and were randomly assigned to a control group given PBS (n = 72) or a treatment group given pasireotide (n = 63) for 9 mo. Mice were also given oral BrdU for 4 wk, commencing at age 20 mo. During the 9 mo from ages 12–21 mo, 25 Men1+/− mice from the PBS-treated group and 12 Men1+/− mice from the pasireotide-treated group died. Thus, 47 Men1+/− mice from the PBS-treated group and 51 Men1+/− mice from the pasireotide-treated group completed the study to age 21 mo, when the mice were reassessed using MRI for changes in pituitary NET volume prior to being culled. Pituitary and pancreatic NETs were collected at necropsy.