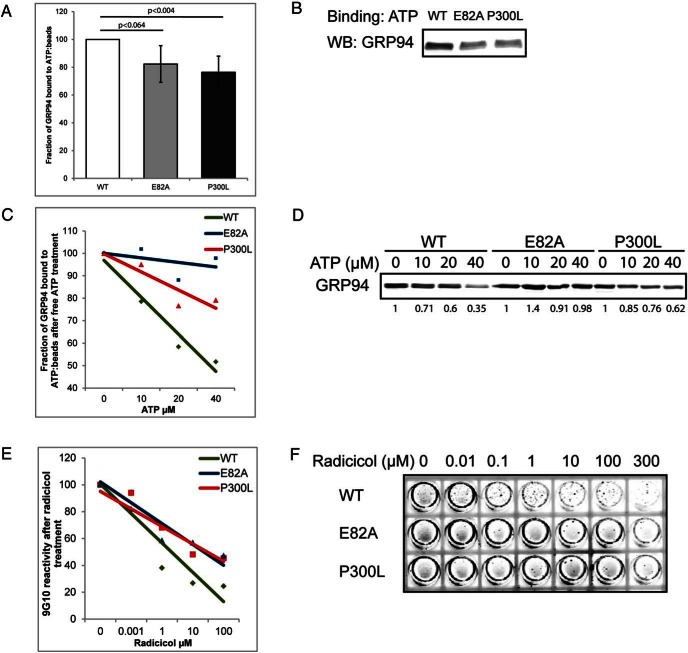
Figure 4.
The P300L mutation affects the nucleotide-binding pocket of GRP94. A–D, His-tagged N34-355 portion of GRP94 was allowed to bind to γ-phosphate-linked ATP resin for 3 hours. Subsequently, aliquots have been analyzed by Western blotting with anti-His antibody to determine the ability of WT and the indicated mutants to bind ATP. Quantitation of eight independent binding experiments with means and SDs are graphed in panel A and one representative experiment shown in panel B. Additionally, the aliquots were incubated with increasing concentrations of free ATP for 3 hours at 4°C. The amount of the remaining ATP-bound GRP94 was analyzed by immunoblotting with anti-His antibody. Trend lines graphs of four independent competition experiments are shown in panel C with one representative experiment shown in panel D. E and F, His-tagged N34-355 GRP94 was bound to Ni-covered plates and preincubated for 4 hours at room temperature with the indicated concentrations of radicicol. Note that most protein was bound to the walls of the wells (circumference). Subsequently, plates were washed and incubated with the anti-GRP94 antibody 9G10 with the same radicicol concentrations for 1 hour. 9G10 binding was detected with an IRDye 800CW-conjugated secondary antibody and scanned on a Licor Odyssey. Trend lines graphs of four independent competition experiments are shown in panel E with one representative experiment shown in panel F.