Figure 1.
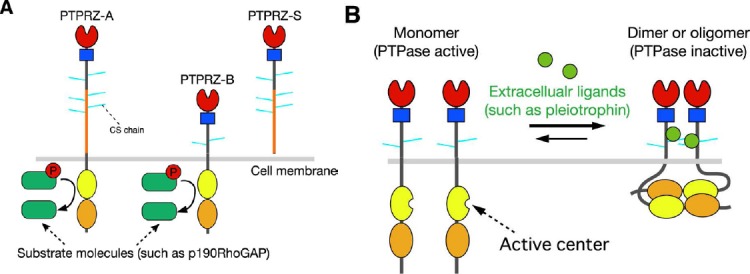
Protein tyrosine phosphatase receptor-type Z (PTPRZ).
(A) Schematic representation of PTPRZ isoforms. The three isoforms expressed in the central nervous system (CNS) are highly glycosylated in the extracellular region by chondroitin sulfate (CS) chains. Domains of the core proteins are highlighted in different colors: CAH (red), carbonic anhydrase-like domain; FNIII (blue), fibronectin type III domain; PTP-D1 (yellow) and PTP-D2 (orange), protein tyrosine phosphatase domain. The catalytic activity is retained in the membrane-proximal PTP-D1, but not in the distal PTP-D2. (B) Ligand-induced dimerization model of PTPRZ. In this model, receptors exist in equilibrium between monomer and dimer conformations. Binding of extracellular ligands including pleiotrophin, midkine, and interleukin-34 induces dimerization (or olimerization) of the receptors, thereby causing the inactivation of the cytoplasmic enzyme. The CS moiety of PTPRZ is important for achieving the high-affinity binding of pleiotrophinto PTPRZ (Maeda et al., 1996; Chow et al., 2008; Kuboyama et al., 2015).
