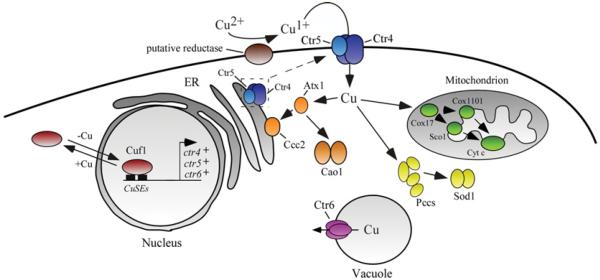
Figure 1. Copper homoeostasis during proliferation in mitosis in S. pombe.
Copper is reduced from Cu2+ to Cu+ by a putative cell-surface reductase. It is then transported across the plasma membrane by a heteroprotein complex formed by the Ctr4 and Ctr5 proteins. There is a mutual dependence between Ctr4 and Ctr5 because trafficking of either protein to the cell surface requires concomitant trafficking of its partner. Results obtained in yeasts S. pombe and S. cerevisiae, in addition to comparison of sequence homology with S. cerevisiae chaperones, suggest that copper chaperones in fission yeast include Atx1, Pccs and Cox17. Atx1 carries copper to Ccc2, a P-type ATPase in the Golgi membrane that pumps copper into the secretory pathway where it can be incorporated into newly synthesized copper proteins. Atx1 is also involved in the delivery of copper to Cao1. In the mitochondrion, Cox17 delivers copper to Cco via Sco1 and presumably Cox1101. Pccs shuttles copper to Sod1 in the cytosol. When the pool of cytoplasmic copper is depleted, Ctr6 participates in copper efflux from the vacuole, providing copper to cytosolic copper-dependent enzymes. In response to copper deficiency, Cuf1 is localized in the nucleus where it binds to CuSEs and activates transport of copper by inducing expression of ctr4+, ctr5+ and ctr6+ genes. In contrast, in cells undergoing a shift from low to sufficient copper concentrations, Cuf1 translocates from the nucleus to the cytoplasm. Brown, putative reductase; blue, Ctr4/Ctr5; orange, Atx1, Ccc2 and Cao1; green, Cox17, Cox1101, Sco1 and cytochrome c oxidase; yellow, Pccs and Sod1; purple, Ctr6; red, Cuf1.