Abstract
BACKGROUND
The guanine nucleotide-binding protein beta polypeptide 3 (GNB3) 825T allele encodes a product that enhances the activation of heterotrimeric G proteins, which is associated with the occurrence of the splice variant Gβ3 s that could play a role in vascular reactivity and hyperproliferation of smooth muscle cells, that makes such proteins attractive candidate gene products for susceptibility to essential hypertension (EH).
OBJECTIVE
To predict the risk for EH in individuals with C825T genetic polymorphism of G protein β3 gene.
METHODS
The study consisted of 222 normotensive individuals and 216 hypertensive patients. Individuals were genotyped for C825T genetic polymorphism of G protein β3 gene rs5443 by using restriction fragment length polymorphism.
RESULTS
Frequencies of C and T alleles were 58.1% and 41.9%, respectively, in the control group compared with 47.7% and 52.3%, respectively, in the hypertensive group. The carriers of rs5443 (T) allele exhibited a significant greater risk for EH compared with the carriers of rs5443 (C) allele (odds ratio = 1.5, 95% confidence interval = 1.2–2.0).
CONCLUSION
T allele is a risk factor for EH in the Egyptian population, which may be used as a prognostic and a therapeutic target of prophylaxis.
Keywords: G protein, polymorphism, essential hypertension, RFLP, PCR, BseDI
Introduction
Both genetic and environmental factors contribute to the pathogenesis of essential hypertension (EH). EH is about twice as common in subjects who have one or two hypertensive parents, and many epidemiological studies suggest that genetic factors account for approximately 30% of the variation in blood pressure in various populations.1 Furthermore, Williams et al reported that premature onset of hypertension among first-degree relatives yielded a remarkable high risk of 3.8 times to develop hypertension.2
Guanine nucleotide-binding proteins (G proteins) are signal transducers that communicate signals from many hormones, neurotransmitters, chemokines, and autocrine and paracrine factors.3 Ligand binding to heptahelical receptors results in the dissociation of the heterotrimeric G protein into Gα-GTP and Gβγ complexes, resulting in various cellular functions.4 Because of their crucial role in the function of many types of cells, genetic abnormalities in G protein subunits have the potential to be involved in the etiology of a wide range of clinical conditions.5 C825T genetic polymorphism is associated with obesity,6 EH,7 depression,8 and cardiovascular diseases.9
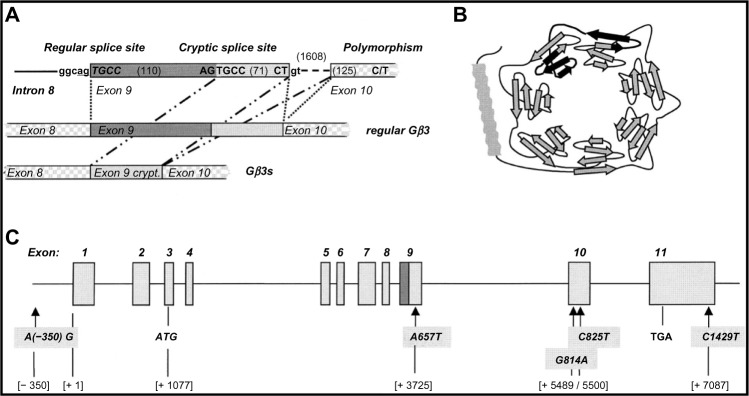
The entire nucleotide-binding protein beta polypeptide 3 (GNB3) gene that is located in chromosome 12p13 spans 7.5 kb and is composed of 11 exons and 10 introns (Fig. 1).5,10 Its promoter lacks TATA box but harbors GC-rich regions.5 All G protein β subunits identified so far consist of seven WD repeats (referring to the conserved amino acids tryptophan “W” and aspartate “D”) that form a regular torus-like structure (Fig. 1).11 WD proteins are found in all eukaryotes but not in prokaryotes and regulate cellular division, cell-fate determination, gene transcription, transmembrane signaling, mRNA modification, and vesicle fusion.12
Figure 1.
GNB3 Gene structure and alternative splicing mechanism. (A) Exons 8, 9, and 10 with the intervening introns. Alternative splicing of exon 9 leads to a deletion (dark gray) in Gβ3s. C/T indicates the C825T polymorphism. (B) Proposed modular structure of Gβ3, which consisting of 7 WD domains. Parts that are deleted in Gβ3s are indicated in black. (C) Gene structure of GNB3.
Notes: Numbered boxes represent exons. ATG and TGA indicate the translation start and stop codons, respectively. Arrows indicate the localization of several polymorphisms in GNB3.
Reused with permission from Rosskopf D, Busch S, Manthey I, Siffert W. G protein β3 gene: structure, promoter, and additional polymorphisms. Hypertension. 2000;36:33–41. Promotional and commercial use of the material in print, digital or mobile device format is prohibited without permission from the publisher, Wolters Kluwer Health (healthpermissions@wolterskluwer.com).
In GNB3, polymorphism C → T (rs5443) at nucleotide number 825 in exon 10 of the β3 subunit of pertussis toxin-sensitive Gi type protein has been identified.13 This polymorphism induces the occurrence of a splice variant in which the nucleotides 498–620 of exon 9 are deleted.14 This deletion causes the loss of 41 amino acids of exon 9 along with the fourth Trp–Asp (WD) of the seven WD repeats that form a propeller structure.15 Such a structure change could alter the position of critical β-propeller residue that contacts the lip in the α-subunit of the αβγ subunit complex, and thus helps the likely GDP exit route, thereby accounting for the enhanced activity of GNB3.5 So, this splice variant is found to be a functional protein and causes enhanced activation of G protein in reconstituted systems.15 Therefore, a higher blood pressure would arise from increased sensitivity to vasoactive pressor hormones known to transmit their signals through Gβ3 proteins.16 In vivo studies confirmed this concept and demonstrated an enhanced vascular reactivity on the stimulation of coronary α1 – adrenergic receptors in carriers of GNB3 825T.17 Likewise, neutrophils from carriers of rs5443T allele exhibit an increased chemotactic response.18
Several studies have evaluated the association between GNB3 C825T polymorphism and EH.19–21 There is a lot of controversy in GNB3 C825T polymorphism in hypertension cases. For novel analysis of the GNB3 polymorphisms, samples from Egyptian subjects are analysed, whom the studies upon them are very limited. Some ethnic groups with this polymorphism have a very strong association with hypertension, whereas some ethnic groups do not. GNB3 polymorphism (C825T) was not associated with EH among Asian population as reported by Guo et al and Li et al.19,20 Another meta-analytic study claims a significant association with GNB3 polymorphism (C825T) in Caucasians, with no detected associations between GNB3 C825T and the risk for overall EH in Asian and Japanese people.21
We aimed to study the risk for EH in the Egyptian population with C825T genetic polymorphism of GNβ3 gene rs5443 to clarify the discrepancies between the ethnic groups that may have a therapeutic impact on these patients, on whom sufficient epidemiological studies on this subject are not available.
Materials and Methods
Subjects
The control group (group I) consisted of 222 normotensive patients (108 males and 114 females) with mean age (38.5 ± 12.4 years). The case group (group II) consisted of 216 hypertensive patients (106 males and 110 females) with mean age (38.6 ± 9.9 years). Total 438 patients’ blood samples were recruited from Ain Shams University Hospitals, from October 2011 to March 2015. All subjects gave their written, informed consent to be included in this study. The study was conducted in the Internal Medicine Department of Ain Shams University Hospitals, and approved by the Research Ethics Committee of Ain Shams University. This research was conducted in accordance with the principles of the Declaration of Helsinki.
Subjects with body mass index (BMI) ≥25 kg/m2 were considered positive for obesity, as defined by the World Health Organization (WHO).22 Subjects were chosen to be matched in age, gender, and BMI between the case and control groups (Table 1).
Table 1.
Demographic data of the studied group.
| PARAMETER | CONTROL (NO. = 222) | CASES (NO. = 216) | P |
|---|---|---|---|
| Mean age ± SD | 38.5 ± 12.4 | 38.6 ± 9.9 | 0.93 |
| Mean BMI ± SD | 26.3 ± 4.1 | 26.7 ± 4.2 | 0.28 |
| Gender | |||
| Male | 48.6% (108) | 49.1% (106) | χ2 (P) |
| Female | 51.4% (114) | 50.9% (110) | 0.008 (0.93) |
The subjects were considered hypertensive if they were currently using antihypertensive drugs or had systolic blood pressure >140 mm Hg or diastolic blood pressure >95 mm Hg.
The clinical information obtained was medical history, current medication use, lifestyle, and anthropometric and blood pressure measurements. The physicians performed a physical examination, and blood samples were collected for laboratory procedures.
DNA extraction
Blood samples that were collected on blood samples collection cards then DNA was extracted from the dried blood spots from the white blood cells by a salting out method,23 using the QIAamp® DNA Blood Mini Kits (Qiagen). Red blood cell lysis was done by using red cell lysis buffer (20 mM Tris-HCl pH 7.6), followed by centrifugation. Nuclei lysis was carried out by cell lysis buffer (10 mM Tris-HCl pH 8.0, 1 mM Ethylene-di-amine-tetra-acetic acid (EDTA) pH 8.0, and 0.1% (w/v) SDS) and 500 µL proteinase K (20 mg/mL), which was kept in a water bath at 56 °C for one hour, and then, protein was precipitated by protein precipitation solution (60 mL of 5M potassium acetate, 11.5 mL of glacial acetic acid, and 28.5 mL of water) followed by centrifugation at low speed (2000 × g) for 10 minutes. Then, the supernatant was removed and placed into a clean Eppendorf tube. Finally, DNA was precipitated by 650 µL isopropanol and inverted to mix. The Eppendorf tube was incubated at room temperature for 15 minutes, followed by centrifugation at full speed (17,000 × g) to recover DNA. Then, the DNA was washed twice with 70% ethanol by centrifugation at 4000 × g after each wash. The DNA pellet was dried in air, rehydrated in TE buffer (pH 7.6), and stored at −20 °C. The DNA concentration and purity were determined by spectrophotometer measurement of absorbances at 260 and 280 nm, respectively.
Polymorphism detection
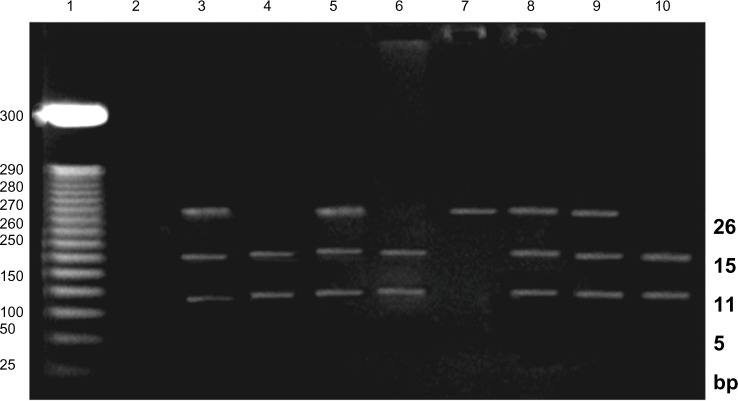
The gene was amplified by polymerase chain reaction (PCR) on 96-well Amp PCR System 9700 Thermocycler (Applied Biosystems). Primer sequences, PCR conditions, and restriction enzyme digestion were as follows (oligonucleotides were synthesized by Promega). The forward primer was 5′-TGACCCACTTGC CACCCGTGC-3′, and the reverse primer was 5′-GCAGCAGCCAGGGCTGGC-3′ (primers accession number: >NC_000012.12). PCR was carried out in a 50-µL reaction volume containing 100 ng of genomic DNA, 0.4 mmol/L of each primer, 0.2 mmol/L dNTPs, 2 mmol/L MgCl2 in 10% PCR buffer, and 1U of DNA polymerase (Promega, UK). PCR involved a first denaturation step of 95 °C for 5 minutes, which was followed by 35 cycles at 94 °C for 1 minute, at 60 °C for 45 seconds, and at 72 °C for 1 minute. The reaction was completed by a final extension step at 72 °C for seven minutes. Aliquots of 5 µL of the PCR products were digested with BseDI (MBI Fermentas). Restriction fragment length polymorphism (RFLP) products were analyzed on 2.5% agarose gel, stained with ethidium bromide, and visualized with ultraviolet transillumination. A 25 base pairs (bp) DNA marker was used, it ranged from 25 to 300 bp (Promega). The CC genotype gave two bands of 115 and 152 bp, CT was expected to give three bands of 267, 152, and 115 bp, while TT gave one band of 267 bp. Figure 2.
Figure 2.
Agarose gel electrophoresis for detection of G protein C825T polymorphism. The CC genotype gave two band of 115 bp, and 152 bp, CT is expected to give three bands 267 bp, 152 bp, and 115 bp, while TT gave one bands of 267 bp. Lane 1 DNA marker, Lane 2: negative control, Lane 3, 5, 8 and 9 (CT) genotype, Lane 4, 6 and 10 (CC) genotype and Lane 7 (TT) genotype. GNB3 Gene structure and alternative splicing mechanism by Rosskopf et al.5 Agarose gel electrophoresis for detection of G protein C825T polymorphism.
Statistical analysis
Genotype and allele frequency were calculated by allele counting as described by Emery.24 Genotype distribution was investigated in relation to Hardy–Weinberg equilibrium that was assessed using a χ2 goodness-of-fit test for biallelic markers (available at http://ihg.gsf.de/cgi-bin/hw/hwa1.pl). Three allele groups were considered for G protein C825T polymorphism rs5443 (CC, CT, and TT). Differences in genotype prevalence and association between the case and control groups were assessed by the chi-square test; odds ratio (OR) and 95% confidence interval (CI) were used to describe the strength of association.24 Mean values for BMI were compared between the different studied groups using Student’s t-test. The nonparametric analysis Kruskal–Wallis test was used for statistical comparison of different genotypes in relation to quantitative variables. Statistical analysis was done with SPSS software version 15.0 (SPSS, Inc).
Results
The distribution of genotypes defined by the C825T polymorphism of GNB3 was examined (Table 2). The frequencies of the rs5443 (C) and rs5443 (T) alleles in our pooled sample were 53% and 47%, respectively. The frequencies of different genotypes CC, CT, and TT in our pooled sample were 28.3%, 49.3%, and 22.4%, respectively.
Table 2.
Frequency of different genotypes of C825T genetic polymorphism among cases and controls (http://bioinfo.iconcologia.net/SNPstats-web).
| GENOTYPE | CONTROL (NO. = 222) | CASES (NO. = 216) | χ2 (P) |
|---|---|---|---|
| CC | 70 (31.5%) | 54 (25%) | 2.3 (0.13) |
| CT | 118 (53.2%) | 98 (45.4%) | 2.65 (0.01*) |
| TT | 34 (15.3%) | 64 (29.6%) | 12.9 (0.00*) |
Note:
P is highly significant (≤0.01).
In the dominant model (Table 3), individuals with (CT and TT) genotype were at much higher risk of developing hypertension (OR = 1.4, 95% CI = 0.9–2.1) when compared with the (CC) genotype. Using the recessive model (Table 4), the risk for hypertension was significantly increased to become twofolds among the carriers of (TT) genotype when compared with the (CC and CT) carriers (OR = 2.3, 95% CI = 1.5–3.7).
Table 3.
Frequency and odds ratio25 of different genotypes of C825T genetic polymorphism among cases and controls: dominant model.
| GENOTYPE | CONTROL (N = 222) | CASES (N = 216) | OR | 95% CI | χ2 (P) |
|---|---|---|---|---|---|
| CC | 70 (31.5%) | 54 (25.0%) | 1 | #0.9–2.1 | 2.3 (0.13) |
| CT and TT | 152 (68.5%) | 162 (75.0%) | 1.4 |
Notes:
Range includes 1 that is not a significant risk factor.
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval.
Table 4.
Frequency and odds ratio25 of different genotypes of C825T genetic polymorphism among cases and control: recessive model.
| GENOTYPE | CONTROL (N = 222) | CASES (N = 216) | OR | 95% CI | χ2 (P) |
|---|---|---|---|---|---|
| CC and CT | 188 (84.7%) | 152 (70.4%) | 1 | *1.5–3.7 | 12.9 (0.0**) |
| TT | 34 (15.3%) | 64 (29.6%) | 2.3 |
Notes:
Range above 1 is a significant risk factor.
P is highly significant (≤0.01).
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval.
Analysis was done based on allele frequencies (Table 5); individuals with rs5443 (T) allele were at much higher risk of developing hypertension compared with the carriers of rs5443 (C) allele (OR = 1.5, 95% CI = 1.2–2.0).
Table 5.
Frequency and odds ratio25 of different alleles of C825T genetic polymorphism among cases and control.
| ALLELE | CONTROL | CASES | OR | 95% CI | χ2 (P) |
|---|---|---|---|---|---|
| C | 258 (58.1%) | 206 (47.7%) | 1 | *1.2–2.0 | 12.183 (0.0**) |
| T | 186 (41.9%) | 226 (52.3%) | 1.5 |
Notes:
Range above 1 is a significant risk factor.
P is highly significant (≤0.01).
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval.
Analysis was done for the frequency of different genotypes of C825T genetic polymorphism rs5443 among different genders, ages and BMIs (Table 6), and ranking test comparing the different genotypes (Table 7).
Table 6.
Frequency of different genotypes of C825T genetic polymorphism rs5443 among different gender, age, and BMI.
| GENOTYPE | CC | χ2 (P) | CT | χ2 (P) | TT | χ2 (P) |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 64 (51.6%) | 0.53 (0.47) | 92 (42.6%) | 6.696 (0.01**) | 58 (59.2%) | 5.39 (0.02*) |
| Female | 60 (48.4%) | 124 (57.4%) | 40 (40.8%) | |||
| Age | ||||||
| ≤40 | 70 (56.5%) | 0.05 (0.82) | 131 (60.6%) | 1.95 (0.163) | 50 (51%) | 2.04 (0.153) |
| >40 | 54 (43.5%) | 85 (39.4%) | 48 (49%) | |||
| BMI | ||||||
| ≤25 | 75 (60.5%) | 21.18 (0.0**) | 87 (40.3%) | 1.43 (0.23) | 27 (27.6%) | 12.5 (0.0**) |
| >25 | 49 (39.5%) | 129 (59.7%) | 71 (72.4%) |
Notes:
P is significant (≤0.05).
P is highly significant (≤0.01).
Table 7.
Ranking test comparing CC, CT, and TT.
| GNB3 | MEAN RANK | χ2 (P) | |
|---|---|---|---|
| Age | CC | 219.98 | 2.6 (0.27) |
| CT | 211.55 | ||
| TT | 236.42 | ||
| BMI Value | CC | 160.41 | 39.43 (0.0**) |
| CT | 237.39 | ||
| TT | 254.85 |
Note:
P is highly significant (≤0.01).
Discussion
In the present study, we investigated the association between a GNB3 825T polymorphism and EH in the Egyptian population. Our study demonstrated a frequency of 47% for T allele among the Egyptian population, which was similar to that reported for Asians (45%).7,26,27 This frequency was higher than that reported for whites elsewhere (28.1%, 95% CI = 26–30.2),14,15,28,29 while it was lower than that reported for black people (79.2%, 95% CI = 76.5–81.9).13,30
This C825T polymorphism is located >1700 bp upstream of the alternative splice site, indicating that the effect of GNB3 825T on splice process is a complex mechanism. However, there are examples that single distant nucleotide exchange, not related to conserved splice branch, donor, and acceptor sites, can cause such alternative splicing.31
Results of examining the association between G protein beta3 subunit C825T genetic polymorphism rs5443 and blood pressure regulation supported the observation that the G protein beta3 subunit gene variant affects renal function. The pathogenesis of the C825T polymorphism relies on the fact that the 825T allele of GNB3 is related to enhanced stimulated G protein activation in cell lines from hypertensive patients.32,33
Polymorphism in several genes has been associated with blood pressure levels.34 One of these genes is G protein. In the present study, carrier of rs5443 (T) allele either in heterozygote or homozygote form (dominant model) were at risk for hypertension (OR = 1.4, 95% CI = 0.9–2.1), the risk was much higher and significant in the recessive model (OR = 2.3, 95% CI = 1.5–3.7). When the study groups were divided into two groups based on the allele distribution, carriers of T allele were at risk of 1.5 for hypertension compared with the carriers of rs5443 (C) allele (OR = 1.5, 95% CI = 1.2–2.0).
Siffert et al had shown the significant association of T allele with EH in Germans.15 After this report, many studies have investigated the association between the C825T polymorphism and hypertension,1,14,35,36 particularly in a study with white population, a positive association had been shown (OR = 1.44, 95% CI = 1.1–2.214 and OR = 2.3, 95% CI = 1.7–3.3).26 In a study done in the Turkish population, T allele had 2.7 times greater risk for hypertension (OR = 2.786, 95% CI = 1.114–6.967, P = 0.028). This association was still significant after adjustment for BMI (OR = 1.78, 95% CI = 1.546–2.074, P = 0.0001).37
Similar results were reported among black population. A threefold higher risk for hypertension was found among the carriers of the T variant in both heterozygotes (OR = 3.43, 95% CI = 0.94–12.4) and homozygotes (OR = 3.87, 95% CI = 1.09–13.8). Association was detected also after adjustment for age, sex, and BMI (OR = 4.14, 95% CI = 1.11–15.4).13
Many studies reported significant associations between the GNB3 C825T polymorphism rs5443 and hypertension, as well as hemodynamic phenotypes such as renal perfusion, LVH, LV diastolic filling, and coronary vasoconstriction and cardiovascular ischemic diseases.7,26,38–40
In the Hypertension and Ambulatory Recording Venetia Study (HARVEST Study), patients carrying the 825T allele had an increased risk for reaching the blood pressure end point (need for antihypertensive therapy) during a mean follow-up of 4.7 years.41 Investigation in an ethnic group from United Arab Emirates demonstrated a strong association of GNB3 825T allele with LVH, but not with EH, and a high prevalence of the TT genotype (27%) and T allele (55%) in patients with EH.42
Furthermore, T allele was associated with obesity, which may affect blood pressure variations.30,43 The association with obesity has been demonstrated in both white and non-white populations.27,30,44 An effect of G protein subunit on adipogenesis has been observed.13,28 Moreover, since increased BMI is associated with increased risk for hypertension, the possibility has been raised that the association of the GNB3 allele with hypertension could be from a primary association with obesity.14 The mechanism to explain T allele and obesity that increased signaling pertussis toxin sensitive G proteins, which have been shown to stimulate adipogenesis.28 Increased Gi activity could also attenuate Gs-mediated lipolysis, leading to impaired adrenergic-mediated lipolysis and obesity.14
Interaction between the 825T polymorphism, obesity status, and physical activity in predicting hypertension in African- Americans was studied by Grove et al.44 They found that homozygotes for the 825T allele who were obese and had a low activity level were 2.7 times more likely to be hypertensive, compared with nonobese, active 825T homozygotes (OR = 2.7, 95% CI = 1.19–6.17, P = 0.02). During physical activity, catecholamines bind to beta-adrenergic receptors and catalyze the mobilization of glucose and fatty acids that are needed to fuel muscle movement.45 The GNB3 C825T polymorphism creates an altered protein product that may negatively affect interaction with beta-adrenergic receptors during physical activity possibly leading to a decrease in response to catecholamines,46 and consequently, to altered energy utilization and adipose homeostasis.47
In meta-analysis studies done in 2013 and 2014, they screened 66 studies regarding hypertension and found that only the allele model showed marginal association with hypertension (OR = 1.07, 95% CI = 1.01–1.13).19,20 Li et al, found a high statistical significance (P = 0.011) for the association between hypertension and TT genotype and T allele in Chinese population, which is in concordance with the Egyptian population in our study.7
Conclusion
In our study, 825T allele was found to be a risk factor for hypertension. Identification of the candidate genes and understanding of their function hold the promise for the establishment of the specific molecular basis for EH. More studies with larger sample sizes on different populations are needed to determine whether suggested functional candidate genes play any role at all in the etiology of EH.
Acknowledgments
Part of this project was published as abstract in the conferences of Federation of American Societies for Experimental Biology (The FASEB Journal. 2012; 26:972.1).
Footnotes
ACADEMIC EDITOR: Karen Pulford, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1067 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the experiments: NSEDH. Analyzed the data: NSEDH. Wrote the first draft of the manuscript: NSEDH. Contributed to the writing of the manuscript: AAM. Agreed with manuscript results and conclusions: NSEDH, AAM, MMA. Jointly developed the structure and arguments for the paper: NSEDH, AAM. Made critical revisions and approved the final version: AAM. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Beevers G, Lip GY, O’Brien E. ABC of hypertension: the pathophysiology of hypertension. BMJ. 2001;322:912–6. doi: 10.1136/bmj.322.7291.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams RR, Hunt SC, Hasstedt SJ, et al. Are there interactions and relations between genetic and environmental factors predisposing to high blood pressure? Hypertension. 1991;18:129–37. doi: 10.1161/01.hyp.18.3_suppl.i29. [DOI] [PubMed] [Google Scholar]
- 3.Neves SR, Ram PT, Lyengar R. G protein pathway. Science. 2002;296:1636–9. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 4.Cabrera-Vera TM, Vanhauwe J, Thomas TO, et al. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24:765–81. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- 5.Rosskopf D, Busch S, Manthey I, Siffert W. G protein β3 gene: structure, promoter, and additional polymorphisms. Hypertension. 2000;36:33–41. doi: 10.1161/01.hyp.36.1.33. [DOI] [PubMed] [Google Scholar]
- 6.Ko KD, Kim KK, Suh HS, Hwang IC. Associations between the GNB3 C825T polymorphism and obesity-related metabolic risk factors in Korean obese women. J Endocrinol Invest. 2014;37(11):1117–20. doi: 10.1007/s40618-014-0182-6. [DOI] [PubMed] [Google Scholar]
- 7.Li XX, A-Yi-Gu-Li YN, Huang JJ, Zhang JP, Ka-Si-Mu-Jiang AX, Ku-Re-Xi YN. C825T polymorphism of G protein beta3 subunit gene and Uygur Hilit type of essential hypertension: a correlation study. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34(3):297–302. [PubMed] [Google Scholar]
- 8.Fang L, Zhou C, Bai S, et al. The C825T polymorphism of the G-protein β3 gene as a risk factor for depression: a meta-analysis. PLoS One. 2015;10(7):e0132274. doi: 10.1371/journal.pone.0132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semplicini A, Grandi T, Sandonà C, Cattelan A, Ceolotto G. G-protein β3-subunit gene C825T polymorphism and cardiovascular risk: an updated review. High Blood Press Cardiovasc Prev. 2015;22(3):225–32. doi: 10.1007/s40292-015-0093-4. [DOI] [PubMed] [Google Scholar]
- 10.Ansari-Lari MA, Muzny DM, Lu J, et al. A gene-rich cluster between CD4 and triosephosphate isomerase genes at human chromosome 12p13. Genome Res. 1996;6:314–26. doi: 10.1101/gr.6.4.314. [DOI] [PubMed] [Google Scholar]
- 11.Clapham DE, Neer EJ. G protein bγ subunits. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 12.Cappuccio FP, Cook DG, Atkinson RW, Wicks PD. The Wandsworth heart and stroke study: a population-based survey of cardiovascular risk factors in different ethnic groups: methods and baseline findings. Nutr Metab Cardiovasc Dis. 1998;8:371–85. [Google Scholar]
- 13.Dong Y, Zhu H, Sagnella GA, Carter ND, Cook DG, Cappuccio FP. Association between the C825T polymorphism of the G-protein β3- subunit gene and hypertension in blacks. Hypertension. 1999;34:1193–6. doi: 10.1161/01.hyp.34.6.1193. [DOI] [PubMed] [Google Scholar]
- 14.Beige J, Hohenbleicher H, Distler A, Sharma AM. G protein beta-3 subunit C 825T variant and ambulatory blood pressure in essential hypertension. Hypertension. 1999;33:1049–51. doi: 10.1161/01.hyp.33.4.1049. [DOI] [PubMed] [Google Scholar]
- 15.Siffert W, Rosskopf D, Siffert G, et al. Association of human G-protein β3 subunit variant with hypertension. Nat Genet. 1998;18:45–8. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 16.Schiffrin EL. Intracellular signal transduction for vasoactive peptides in hypertension. Can J Physiol Pharmacol. 1994;72:954–62. doi: 10.1139/y94-133. [DOI] [PubMed] [Google Scholar]
- 17.Baumgart D, Naber C, Haude M, Oldenburg O, Erbel R. G protein β3- subunit 825 allele and enhanced coronary vasoconstriction on α2-adrenoceptor activation. Circ Res. 1999;85:965–9. doi: 10.1161/01.res.85.10.965. [DOI] [PubMed] [Google Scholar]
- 18.Virchow S, Ansorge N, Rosskopf D, Rubben H, Siffert W. The G protein β3-subunit splice variant Gβ3-s causes enhanced chemotaxis of human neutrophils in response to interleukin−8. Naunyn Schmiedebergs Arch Pharmacol. 1999;369:27–32. doi: 10.1007/s002109900040. [DOI] [PubMed] [Google Scholar]
- 19.Guo L, Zhang LL, Zheng B, Liu Y, Cao XJ, Pi Y, et al. The C825T polymorphism of the G-protein β3 subunit gene and its association with hypertension and stroke: an updated meta-analysis. Plos One. 2013 Jun 14;8(6) doi: 10.1371/journal.pone.0065863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Zhang B1, Li C1, et al. G-protein beta 3 subunit polymorphisms and essential hypertension: a case-control association study in northern Han Chinese. Journal of Geriatric cardiology. 2015;12:127–34. doi: 10.11909/j.issn.1671-5411.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng H, Xu H, Cui B, Xie N, Wang Z, Luo M. Association between polymorphism of the G-protein β3 subunit C825T and essential hypertension: an updated meta-analysis involving 36,802 subjects. Biol Res. 2013;46:265–73. doi: 10.4067/S0716-97602013000300007. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization . Geneva. World Health Organization; Geneva: 1998. Jun 3–5, 1997. Obesity: preventing and managing the global epidemic report of a WHO consultation on obesity. [PubMed] [Google Scholar]
- 23.Josef S, David WR, Nina I, Kaaren AJ. Molecular Cloning: Rapid Isolation Of Mammalian DNA. Cold Spring Harbour Laboratory Press; New York: 2002. pp. 628–30. [Google Scholar]
- 24.Emery AEH. Methodology in medical genetics - an introduction to statistical methods. Edinburgh: Longman Group Ltd; 1986. [Google Scholar]
- 25.Bland JM, Altman DG. Statistics Notes: The odds ratio. BMJ. 2000;320:1468. doi: 10.1136/bmj.320.7247.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato N, Sugiyama T, Morito H, Kurihara H, Yamori Y, Yazaki Y. G protein ß3 subunit variant and essential hypertension in Japanese. Hypertension. 1998;32:935–8. doi: 10.1161/01.hyp.32.5.935. [DOI] [PubMed] [Google Scholar]
- 27.Siffert W, Forster P, Jöckel K-H, Mvere DA, Brinkmann B, Naber C, et al. Worldwide ethnic distribution of the G protein β3 subunit 825T allele and its association with obesity in Caucasians, Chinese, and black African individuals. J Am SocNephrol. 1999;10:1921–30. doi: 10.1681/ASN.V1091921. [DOI] [PubMed] [Google Scholar]
- 28.Benjafield AV, jeyasingam CI, Nyholt DR, Griffiths LR, Morris BJ. G protein ß3 subunit gene (GNB3) variant in causation of essential hypertension. Hypertension. 1998;32:1094–7. doi: 10.1161/01.hyp.32.6.1094. [DOI] [PubMed] [Google Scholar]
- 29.Brand E, Hermann S-M, Nicaud V, Ruidavets JB, Evans A, Arveiler D, et al. The 825 C/T polymorphism of the G-protein ß3 is not related to hypertension. Hypertension. 1999;33:1175–8. doi: 10.1161/01.hyp.33.5.1175. [DOI] [PubMed] [Google Scholar]
- 30.Iiri T, Farfel Z, Bourne HR. G-protein diseases furnish a model for the turn-on switch. Nature. 1998;394:35–8. doi: 10.1038/27831. [DOI] [PubMed] [Google Scholar]
- 31.Siffert W, Rosskopf D, Moritz A, Wieland T, Kaidenberg-Stasch S, Kettler N, et al. Enhanced G protein activation in immortalized lymphoblasts from patients with essential hypertension. J Clin invest. 1995;96:759–66. doi: 10.1172/JCI118120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alioglu E, Ercan E, Tengiz I, Yidiz A, Turk UO, Berdeli A. The relationship between alpha-adducin polymorphism and non-dipper phenomenon in essential hypertension. J Card Resc. 2007;4:58–67. [Google Scholar]
- 33.Timberlake DS, O’Connor DT, Pamer RJ. Molecular genetics of essential hypertension:recent results and emerging strategies. CurrOpinNephrolhypertens. 2001;10:71–9. doi: 10.1097/00041552-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Hayakawa T, Takamura T, Abe T, Kaneko S. Association of the C825T polymorphism of G-protein beta 3 subunit gene with hypertension, obesity, hyper-lipidemia, insulin resistance, diabetes, diabetic complications, and diabetic therapies among Japanese. Metabolism. 2007;56:44–8. doi: 10.1016/j.metabol.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Casiglia E, Tikhonoff V, Caffi S, Martini B, Guidotti F, bolson M, et al. Effects of the C825T polymorphism of GNB3 gene on body adiposity and blood pressure in fertile and menopausal women: a population-based study. J Hypertens. 2008;26:238–43. doi: 10.1097/HJH.0b013e3282f2b90c. [DOI] [PubMed] [Google Scholar]
- 36.Alioglu E, Ercan E, Tengiz I, Yidiz A, Turk UO, Berdeli A, et al. G protein beta 3 subunit gene polymorphism in Turkish hypertensives. The americam Journal of Cardiology. 2008;8:331–5. [PubMed] [Google Scholar]
- 37.Schunkert H, Hense HW, Doring A, Riegger GA, siffert W. Association between a polymorphism in the G protein beta 3 subunit gene and lower renin and elevated diastolic blood pressure levels. Hypertensionl. 1998;32:510–3. doi: 10.1161/01.hyp.32.3.510. [DOI] [PubMed] [Google Scholar]
- 38.Baumgart D, Naber C, Haude M, Oldenburg O, Erbel R, Heusch G, et al. G protein beta 3 subunit 825T allele and enhanced coronary vasoconstriction on alpha 2 adrenoceptor activation. Circ Res. 1999;85:965–9. doi: 10.1161/01.res.85.10.965. [DOI] [PubMed] [Google Scholar]
- 39.Poch E, Gonzalez D, Gomez-Angelats E, Enjuto M, Pare JC, Rivera F, et al. G-protein beta3 subunit gene variant and left ventricular hypertrophy in essential hypertension. Hypertension. 2000;35:214–8. doi: 10.1161/01.hyp.35.1.214. [DOI] [PubMed] [Google Scholar]
- 40.Saroti M, Semplicini A, Siffert W, Mormino P, Mazzer A, Pegoraro F, et al. G-protein beta3-subunit gene 825T allele and hypertension: a longitudinal study in young grade I hypertension: a longitudinal study in young grade I hypertensives. Hypertension. 2003;42:909–14. doi: 10.1161/01.HYP.0000097600.58083.EE. [DOI] [PubMed] [Google Scholar]
- 41.Mahmood MS, Mian ZS, Afzal A, Frossard PM. G-protein beta 3 subunit gene 825C>T dimorphism is associated with left ventricular hypertrophy but not essential hypertension. Med SciMonit. 2005;11:CR6–9. [PubMed] [Google Scholar]
- 42.Siffert W, Naber C, Walla M, Ritz E. G protein β3 subunit 825T allele and its potential association with obesity in hypertensive individuals. J Hypertens. 1999;17:1–4. doi: 10.1097/00004872-199917080-00008. [DOI] [PubMed] [Google Scholar]
- 43.Hegele RA, Anderson C, Young TK, Connelly PW. G-protein β3 subunit gene splice variant and body fat distribution in Nunavut Inuit. Genome Res. 1999;9:972–7. doi: 10.1101/gr.9.10.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grove ML, Morrison A, Folsom AR, Boerwinkle E, Hoelscher Bray MS. Gene–environment interaction and GNB3 gene in the atherosclerosis. Risk in Communities Study. 2007;31:919–26. doi: 10.1038/sj.ijo.0803545. [DOI] [PubMed] [Google Scholar]
- 45.lodish H, Berk A, Zipursky LS, Matsudaira P, Baltimore D, Darnell J. Cell-to-cell signaling: hormones and receptors. In: Tenney S, editor. Molecular Cell Biology. WH freeman; New York: 2000. [Google Scholar]
- 46.Hauner H, Roting K, Siffert W. Effects of the G-protein β3 subunit 825T allele on adipogenesis and lipolysis in cultured human preadipocytes and adipocytes. HormMetab Res. 2002;34:475–80. doi: 10.1055/s-2002-34786. [DOI] [PubMed] [Google Scholar]
- 47.Ryden M, Faulds G, Hoffstedt J, Wennlund A, Amer P. Effect of the (C825T) Gβ3 polymorphism on adrenoceptor-mediated lipolysis in human fat cells. Diabetes. 2002;51:1601–8. doi: 10.2337/diabetes.51.5.1601. [DOI] [PubMed] [Google Scholar]