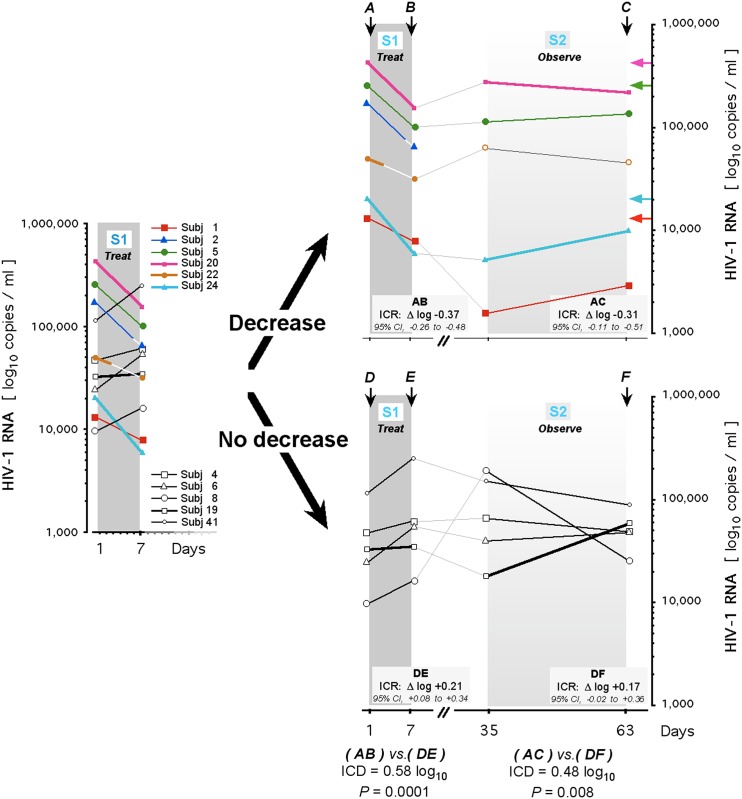
Fig 8. Persistent HIV-1 suppression after deferiprone cessation in treatment-naive HIV-infected subjects.
Discontinuation trial design (DTD) is used to analyze long-term rebound following HIV-1 RNA—based segregation into a ‘Decrease’ and a ‘No decrease’ cohort, defined by viral load post-drug on Day 7 relative to viral load pre-drug on Day 1. S1, first stage of protocol (one-week treatment); S2, second stage of protocol (eight-week observation). Left: HIV-1 RNA levels in each trial subject immediately before and after the one-week treatment period (S1). Subject 22 discontinued oral intake on Day 3 (after the 7th dose) and Subject 2 discontinued oral intake on Day 5 (after the 13th dose), as indicated by the white line segments (for clinical details, see Results). Right: Presence or absence of a decrease in HIV-1 RNA after the one-week treatment period (S1) segregates subject into the two subsets for the eight-week observation period (S2). Horizontal arrows (at C in S2 of upper panel) delineate the pre-drug viral load on Day 1 (at A in S1 of upper panel), color-coded to an individual’s post-drug viral load on Day 35 and Day 63 (28 and 56 days after drug cessation). Subject 2 did not enter S2 analysis. Subject 22 did enter S2 analysis and reacquired the pre-medication viral load at 4 weeks post-treatment, verified at 8 weeks post treatment (open circles, S2 of upper panel). A and D, HIV-1 RNA copies immediately before the intake of the first dose of deferiprone on Day 1; B and E, HIV-1 RNA copies immediately after the last dose of deferiprone on Day 7; C and F, HIV-1 RNA copies on Day 63 of protocol, i.e. day 56 off-drug. Two-letter combinations indicate the period of the intra-cohort response (ICR). Extent and significance of the inter-cohort differences (ICDs) are indicated for the identified periods.