Fig. 3. Specific regulation of Gα subunits by a 7TM-RGS supports the notion of coevolution of Gα subunits with receptor GAPs.
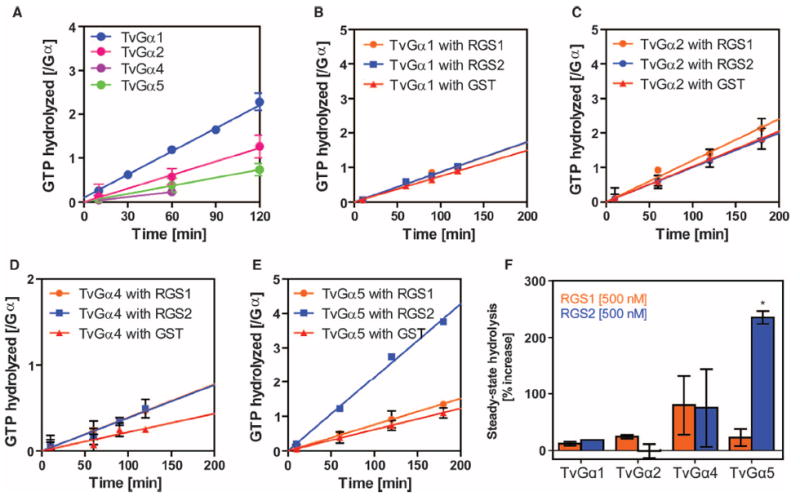
(A) Steady-state hydrolysis of GTP by the indicated wild-type T. vaginalis Gα (TvGα) subunits. Purified Gα subunits (250 or 500 nM) were incubated with 10 μM [γ-32P]GTP for the indicated times before hydrolyzed 32PO4 was extracted with charcoal and quantified. All of the wild-type T. vaginalis Gα subunits displayed relatively slow rates of intrinsic hydrolysis. Data show the relative amounts of GTP hydrolyzed per mole of Gα subunit from two independent experiments. (B to F) Effects of RGS proteins on the GTP hydrolysis rates of TvGα subunits. The indicated Gα subunit (250 nM) and the indicated RGS proteins (500 nM) were incubated over a (B and D) 2-hour or (C and E) 3-hour period. Steady-state [γ-32P]GTP hydrolysis rates were calculated for (B) TvGα1, (C) TvGα2, (D) TvGα4, and (E) TvGα5 in the presence of 500 nM RGS1, RGS2, or glutathione S-transferase (GST). (F) Summary. Enhancement of the GTPase activities of the indicated TvGα subunits by RGS1 or RGS2 relative to those in the presence of the GST control. GAP activity was only seen with RGS2 on TvGα5. *P < 0.01, analysis of variance (ANOVA) followed by Tukey’s test. All other combinations led to statistically insignificant changes in GTPase activity. All data are representative of at least two experiments. Error bars represent SD.
