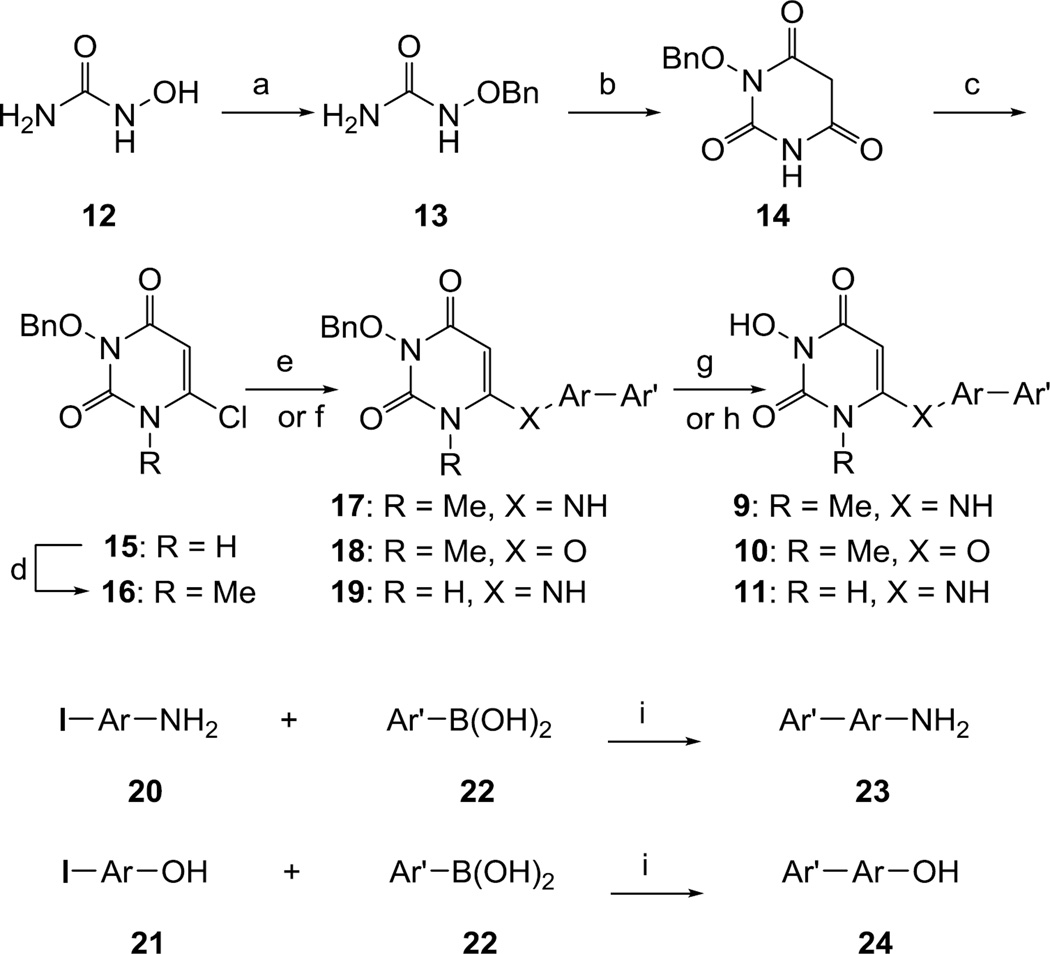
Scheme 1a.
Synthesis of HPD analogues 9–11.
a Reagents and conditions: a) KOH, BnCl, MeOH, reflux, 6 h, 91%; b) CH2(COOEt)2, NaOEt, MW, 150 °C, 20 min, 58%; c) POCl3, BnEt3NCl, 50 °C, 6 h, 88%; d) Cs2CO3, MeI, DMF, seal tube 80 °C, 2h, 68 %; e) (for 17–18) Ar′-ArNH2/ Ar′-ArOH, LDA, HMPA, THF, −78 °C to rt, overnight; f) (for 19) Ar′-ArNH2, N,N-dimethylaniline, MW, 170 °C, 30–40 min; g) Pd/C, H2, 50 Psi, MeOH, 3–4h; h) TFA, MW, 120 °C, 30 min; i) Pd(PPh3)4 1.5 % (mol), DMF, 2M Na2CO3, MW, 160 °C, 30 min.