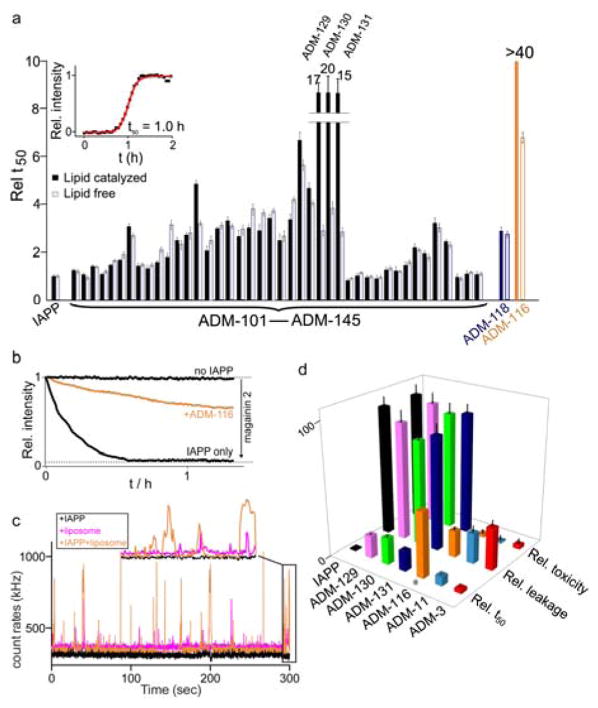
Fig. 2.
Effects of small molecules on processes mediated by IAPP. (a) Reaction mid-points, t50, of aqueous (white) and lipid-catalysed (black) fibrillation reactions. The t50 are expressed relative to reference reactions in which the indicated compound is absent. Inset: Representative lipid–catalysed kinetic profile (black) and sigmoidal fit (red) used to extract t50. Liposome catalysed reaction conditions: 10 μM IAPP, 10 μM oligoquinoline, DOPG:DOPC (1:1, 630 μM, d = 100 nm). Fully aqueous condition: 30 μM IAPP, 6 μM oligoquinoline. (b) Representative kinetic profiles for liposome leakage mediated by IAPP in the presence and absence of ADM-116. The dynamic range for renormalization is established using 20 μM magainin 2. Membrane leakage assayed using 100 nm, DOPG liposomes at 200 μM lipid in monomer units, 6 μM IAPP and stoichiometric oligoquinoline. (c) Representative burst profile of the diffusion of 20 nM fluorescently labelled ADM-116 through the excitation volume of a confocal microscope. Shown are 1 μM IAPP (black), 25 μM liposomes (magenta) or both (orange). Inset shows an expansion of the final 10 s of the trace. (d) Statistics for the effects observed using the indicated molecules in our primary, and two indicated secondary assays (see main text). All values are expressed as renormalized averages relative to IAPP-only controls. Cytotoxicity measured at 13 μM IAPP and stoichiometric small molecule. oligoquinoline. Cytotoxicity was measured using cell-titer blue colorimetric assay. Error bars for toxicity assay represent standard deviations from a minimum of three independent experiments each with four internal repeats (i.e. n ≥ 12).