Abstract
Purpose
Existing data regarding the expression of estrogen receptors (ER) and prostate cancer outcomes have been limited. We evaluated the relationship of expression profiles of ERβ subtypes and the estrogen receptor GPR30 with patient factors at diagnosis and outcomes following radical prostatectomy.
Materials and Methods
Tissue microarrays constructed from 566 men with long-term clinical follow-up were analyzed with immunohistochemistry targeting ERβ1, ERβ2, ERβ5 and GPR30. An experienced pathologist scored receptor distributions and staining intensities. Tumor staining characteristics were evaluated for associations with patient characteristics, recurrence free survival, and prostate cancer-specific mortality (PCSM) following radical prostatectomy. Results: Prostate cancer cells had unique receptor subtype staining patterns with ERβ1 demonstrating predominantly nuclear localization, while ERβ2, ERβ5 and GPR30 were predominantly cytoplasmic. After controlling for patient factors, intense cytoplasmic ERβ1 staining was independently associated with time to recurrence (HR 1.7, 95% CI 1.1-2.6, p=0.01) and PCSM (HR 6.6, 95% CI 1.8-24.9, p=0.01). Similarly, intense nuclear ERβ2 staining was independently associated with PCSM (HR 3.9, 95% CI 1.1-13.4, p=0.03). Patients with cytoplasmic ERβ1 and nuclear ERβ2 co-staining had significantly worse 15-year PCSM vs. patients expressing only cytoplasmic ERβ1, only nuclear ERβ2, or neither (16.4% vs. 4.3% vs. 0.0% vs 2.0 %, respectively p=0.001).
Conclusions
Increased cytoplasmic ERβ1 and nuclear ERβ2 expression are associated with worse cancer-specific outcomes following radical prostatectomy. These findings suggest that tumor ERβ1 and ERβ2 staining patterns provide prognostic information for radical prostatectomy patients.
Keywords: Prostate Cancer, Estrogen Receptor, Prostatectomy
Introduction
Prostate cancer (PCa) is the second leading cause of male cancer-specific mortality in the United States, with approximately 30,000 deaths annually.1 Although many men die from PCa, the majority of men diagnosed in the PSA era have low-risk disease from which many never develop symptoms of their disease irrespective of treatment. This wide spectrum of disease behavior underscores the need for biomarkers of PCa-specific outcomes to accurately predict PCa prognosis and tailor treatment to each patient's disease.
One potential biomarker is estrogen activity, whose role in prostate carcinogenesis and treatment has been evaluated extensively.2 The main effectors of estrogen signaling are the estrogen receptors (ER)3, of which ERβ is the dominant receptor in the prostate epithelium.4 ERβ has been hypothesized to play an anti-proliferative role within the prostate based on the inhibition of cellular proliferation in PCa cells,5 the development of hyperplasia and dysplasia in ERβ knockout mice,6,7 the reduction in ERβ expression in high-grade intraprostatic neoplasia vs. benign glands,8,9 and the marked decrease in ERβ expression in various high vs. low-grade tumors.8,10 However, ERβ expression has been observed in the majority of nodal and boney metastases8,9 and has been associated with worse overall survival among hormone naïve patients initiating androgen deprivation therapy. 11
Recent identification of ERβ isoforms provides insights into the complex biological outcomes. In addition to the wild-type (WT) ERβ (ERβ1), humans have four splice variants, ERβ2-5.12 ERβ1 is the only fully functional receptor, while ERβ2, β4 and β5 heterodimerize with ERβ1 regulating its transactivation.13 The function of ERβ isoforms as mediators of estrogen signaling suggests that ERβ isoform expression patterns could influence the biology of malignant cells and in turn, have a prognostic role14.
We evaluated the relationship between ERβ isoform expression patterns and oncologic outcomes in men with localized PCa treated with radical prostatectomy (RP). Additionally, based on recent data suggesting that stimulation of the estrogen-binding G-protein-coupled receptor-30 (GPR30) inhibits the growth of PCa both in vitro and in vivo in murine xenografts15 we also evaluated the association between GPR30 expression and post-RP outcomes.
Methods
Study Population
We performed a retrospective review of men who underwent RP for histologically confirmed clinically localized PCa and were previously enrolled in population-based studies of PCa in King County, WA16,17 The first study ascertained cases under age 65 years who were diagnosed between 1993 and 1996, and for the second study, men were under age 75 and were diagnosed between 2002 and 2005. These studies included N=831 patients who were identified from the Seattle-Puget Sound SEER cancer registry and underwent structured in-person interviews conducted by trained staff to collect demographics and past medical history data as previously described.16,17 Formalin-fixed paraffin embedded (FFPE) blocks of tumor tissue were available in N=566 interviewed patients and made up the present study population. Follow-up surveys were completed by patients between 2004 and 2005 and 2010 and 2011. Clinical data including Gleason score, pathologic stage, PSA at diagnosis, and primary therapy were obtained from the Seattle-Puget Sound SEER cancer registry. Vital status as of December 2013 and underlying cause of death were determined through linkage with the SEER registry and review of death certificates. Recurrence was defined as PSA ≥ 0.2 ng/mL; positive bone scan, computed tomography, and/or magnetic resonance imaging; positive lymph node or prostate bed biopsy; receipt of secondary or salvage therapies; physician statement of PCa recurrence, spread and/or PCa-specific mortality (PCSM) following RP. The study received approval from the Fred Hutchinson Cancer Research Center Institutional Review Board and all patients provided informed consent.
Construction of Tissue Microarrays and Immunohistochemistry
Hematoxylin and eosin slides were made from FFPE blocks of tumor tissue obtained at the time of RP. An experienced genitourinary pathologist (X.Z.) reviewed the slides and marked regions containing ≥75% tumor. Duplicate 1.0 mm diameter cores were taken from the dominant tumor focus in the corresponding region of the block and arrayed into a new recipient paraffin block. Five-micron tissue microarray (TMA) sections were then cut, deparaffinized, and rehydrated in dH2O. TMA immunohistochemistry was performed for the detection of ERβ1, ERβ2, ERβ5, and GPR30.14 The dilution ratio for the primary antiserum was 1:100 for ERβ1 and ERβ5, and 1:500 for ERβ2.14,18 Anti-GPR30 antibody (ab12563) was purchased from Abcam (Cambridge, MA) and the dilution ratio was 1:100.
The immunostaining of each receptor within the cancerous glands was scored by an experienced pathologist (X.Z.) blinded to clinical parameters. Cytoplasmic and nuclear staining were evaluated separately. Tissue cores with unsatisfactory staining, uncertain histology, or that were missing/damaged were excluded from the analysis. Intra-observer concordance was evaluated by rescoring a randomly selected 2% sample by the study pathologist. Intra-patient concordance was evaluated on each of the two core samples from the same 2% sample. As previously described,19 immunostaining was assessed using a score created by multiplying staining intensity (0 for no staining, 1 for light staining, and 2 for strong staining) by the corresponding percentage of cells staining positive at each intensity. The mean score was used for cases with data from duplicate cores. Based on the distribution of staining intensities, weak staining was defined as a staining score of >0 to <1 and intense staining as a staining score ≥1.
Statistical Analysis
Associations between cytoplasmic and nuclear ERβ1, ERβ2, ERβ5, and GPR30 staining and clinicopathologic data were analyzed with the Chi2 test. Associations between receptor staining profiles and PCa outcomes were evaluated with the Kaplan-Meier method and the log-rank test. Receptors found to be significantly associated with time to recurrence or PCSM were then evaluated in multivariable Cox proportional hazards models adjusted for age, diagnostic PSA, Gleason score, and pathologic stage. Significance was set at <0.05, two-tailed test. All analyses were performed with Stata SE/12 (College Station, TX).
Results
Descriptive statistics for the study population are outlined in Table 1. The median diagnostic PSA was 6.0 (± 10.2) ng/mL, 290 (51.2%) men had ≥ Gleason grade 7, and 390 (68.7%) had organ-confined (pT2) disease (Table 1). Among survivors, the median follow-up was 10.5 years (range 0.7 – 20.3 years).
Table 1. Selected characteristics of the prostate cancer patient cohort.
| Characteristic | N (%) |
|---|---|
| Age (y) at diagnosis | |
| 35-49 | 71 (12.5) |
| 50-54 | 104 (18.4) |
| 55-59 | 138 (24.4) |
| 60-64 | 164 (29.0) |
| 65-69 | 57 (10.1) |
| 70-74 | 32 (5.6) |
| Race | |
| European- American | 519 (91.7) |
| African-American | 47 (8.3) |
| BMI | |
| <25 | 180 (31.8) |
| 25-29.9 | 286 (50.5) |
| 30+ | 100 (17.7) |
| Pathological Stage | |
| Localized | 390 (68.9) |
| Regional | 176 (31.1) |
| Gleason Sum | |
| 2-6 | 276 (48.8) |
| 7 (3+4) | 202 (35.7) |
| 7 (4+3)-10 | 88 (15.5) |
| PSA at diagnosis (ng/mL) | |
| 0-3.9 | 82 (14.5) |
| 4-9.9 | 338 (59.7) |
| 10-19.9 | 76 (13.4) |
| 20+ | 35 (6.2) |
| Missing | 35 (6.2) |
| Recurrence | |
| No | 341 (60.3) |
| Yes | 119 (21) |
| Unknown | 106 (18.7) |
| Vital Status | |
| Alive | 495 (87.5) |
| Prostate cancer death | 13 (2.3) |
| Other cause of death | 53 (9.3) |
| Unknown cause of death | 5 (0.9) |
Unique expression was observed for each receptor (Figures 1 and 2). Tumors expressed ERβ1 in 95.7%, ERβ2 in 97.5%, GPR30 in 97.5%, and ERβ5 in 22.9% of interpretable patients. ERβ1 staining was nuclear only in 316 (62.1%), cytoplasmic only in 43 (8.5%), and in both the nucleus and cytoplasm in 128 (25.1%) patients. ERβ2 staining was nuclear only in 5 (1.0%), cytoplasmic only in 376 (72.0%), and in both in 129 (23.7%) patients. Conversely, ERβ5 and GPR30 demonstrated minimal heterogeneity with nearly exclusive cytoplasmic staining. Intra-observer and intra-patient concordance were ≥85% for all receptors. Associations between receptor staining and patient clinicopathologic data are presented in Table 2. The staining distribution of cytoplasmic ERβ1 (cERβ1) was significantly different (p=0.04) between patients with localized disease (none: 67.5%, weak: 11.9%, intense: 20.5%) vs. regional disease (none: 64.0%, weak: 6.7%, intense: 29.2%). No associations were observed between clinical data and ERβ2, ERβ5, or GPR30 staining.
Figure 1.
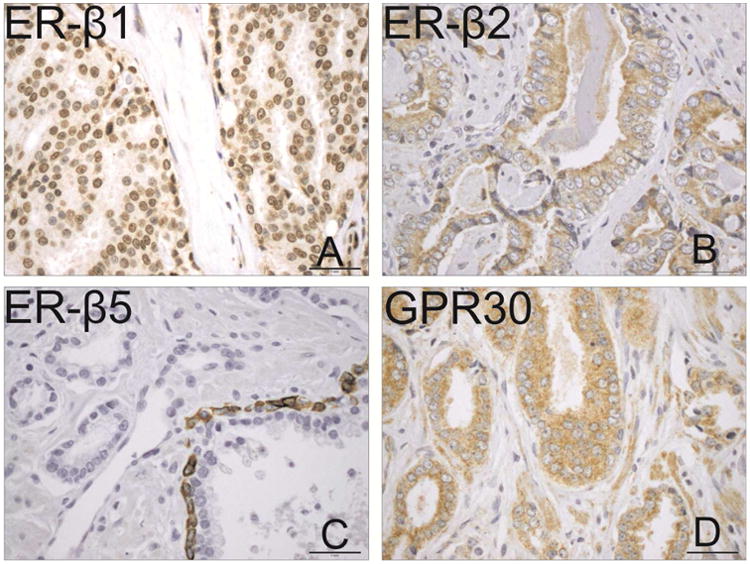
Representative immunohistochemical staining of ERβ1 (A), ERβ2 (B), ERβ5 (C) and GPR30 (D) at 100× magnification.
Figure 2.
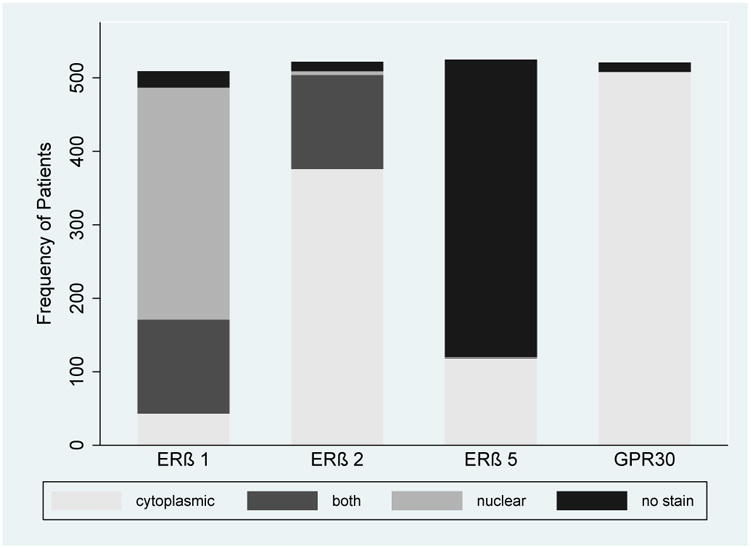
Distribution of estrogen receptor staining (ERβ1, ERβ2, ERβ5 and GPR30) by location (any vs. no staining).
Table 2. Association between selected clinicopathologic characteristics and estrogen receptor cERβ1 and nERβ2 staining in prostate cancer patients.
| cERβ1 | nERβ2 | |||||||
|---|---|---|---|---|---|---|---|---|
| None | Weak | Intense | None | Weak | Intense | |||
|
|
||||||||
| N (%) | N (%) | N (%) | p-value* | N (%) | N (%) | N (%) | p-value* | |
| 338 (66.4) | 52 (10.2) | 119 (23.4) | 387 (74.4) | 81 (15.6) | 52 (10.0) | |||
|
|
||||||||
| Age | ||||||||
| 35-49 | 48 (14.2) | 5 (9.6) | 10 (8.4) | 0.19 | 49 (12.7) | 11 (13.6) | 5 (9.6) | 0.5 |
| 50-54 | 60 (17.8) | 10 (19.2) | 26 (21.8) | 80 (20.7) | 13 (16.1) | 5 (9.6) | ||
| 55-59 | 78 (23.1) | 20 (38.5) | 30 (25.2) | 95 (24.5) | 19 (23.5) | 15 (28.8) | ||
| 60-64 | 94 (27.8) | 14 (26.9) | 38 (31.9) | 105 (27.1) | 24 (29.6) | 21 (40.4) | ||
| 65-69 | 39 (11.5) | 2 (3.8) | 8 (6.7) | 39 (10.1) | 7 (8.6) | 4 (7.7) | ||
| 70-74 | 19 (5.6) | 1 (1.9) | 7 (5.9) | 19 (4.9) | 7 (8.6) | 2 (3.9) | ||
| Race | ||||||||
| European-American | 306 (90.5) | 51 (98.1) | 108 (90.8) | 0.19 | 353 (91.2) | 76 (93.8) | 47 (90.4) | 0.71 |
| African-American | 32 (9.5) | 1 (1.9) | 11 (9.2) | 34 (8.8) | 5 (6.2) | 5 (9.6) | ||
| BMI | ||||||||
| <25 | 104 (30.8) | 16 (30.8) | 39 (32.8) | 0.61 | 127 (32.8) | 21 (25.9) | 16 (30.8) | 0.41 |
| 25-30 | 166 (49.1) | 27 (51.9) | 64(53.8) | 185 (47.8) | 48 (59.3) | 28 (53.8) | ||
| >30 | 68 (20.1) | 9 (17.3) | 16 (13.4) | 75 (19.4) | 12 (14.8) | 8 (15.4) | ||
| Stage | ||||||||
| Localized | 233 (68.9) | 41 (78.8) | 71 (59.7) | 0.04 | 266 (68.7) | 55 (67.9) | 30 (57.7) | 0.28 |
| Regional | 105 (31.1) | 11 (21.2) | 48 (40.3) | 121 (31.3) | 26 (32.1) | 22 (42.3) | ||
| Gleason Sum | ||||||||
| 2-6 | 165 (48.8) | 26 (50.0) | 51 (42.9) | 0.1 | 190 (49.1) | 38 (46.9) | 21(40.4) | 0.65 |
| 7 (3+4) | 130 (38.5) | 15(28.8) | 42 (35.3) | 142 (36.7) | 29 (35.8) | 20(38.5) | ||
| 7 (4+3)-10 | 43 (12.7) | 11 (21.2) | 26 (21.8) | 55 (14.2) | 14 (17.3) | 11(21.1) | ||
| PSA at diagnosis (ng/dL) | ||||||||
| 0-3.9 | 54 (16) | 7 (13.5) | 17 (14.3) | 0.21 | 53 (13.7) | 10 (12.3) | 13(25.0) | 0.38 |
| 4-9.9 | 202 (59.8) | 28 (53.8) | 68 (57.1) | 235 (60.7) | 48 (59.3) | 25(48.1) | ||
| 10-19.9 | 49 (14.5) | 8 (15.4) | 15 (12.6) | 50 (12.9) | 13 (16.1) | 8 (15.4) | ||
| 20+ | 14 (4.1) | 6 (11.5) | 12 (10.1) | 23 (5.9) | 6 (7.4) | 4 (7.7) | ||
| Unknown | 19 (5.6) | 3 (5.8) | 7 (5.9) | 26 (6.7) | 4 (4.9) | 2 (3.8) | ||
Univariate, Chi2
Clinical Outcomes
PCa recurrence information was available on 460 patients (81.3%, 18.7% of patients did not return follow-up questionnaires) in which there were 119 (25.9%) recurrences, correlating to a 5-year and 10-year recurrence free survival (RFS) probability of 85.0% and 72.6% following RP. On Kaplan-Meier analysis (Figure 3), cERβ1 staining intensity was significantly associated with shorter time to recurrence (p=0.004) with 5-year recurrence free survival (RFS) probabilities of 73.2% for intense vs. 91.1% for weak vs. 88.3% for no staining. nERβ2 staining intensity was not significantly associated with time to recurrence (p=0.11), however, when censored at 5-years intense nERβ2 staining intensity was associated with significantly worse 5-year RFS compared to weak or no staining (69.1 % vs. 81.4% vs. 88.1%, p=0.02). On multivariable analysis, intense cERβ1 staining was independently associated with time to recurrence (HR 1.7, 95% CI 1.1 – 2.6), p=0.01), when adjusting for patient age, Gleason score, pathologic stage and diagnostic PSA (Table 3).
Figure 3.
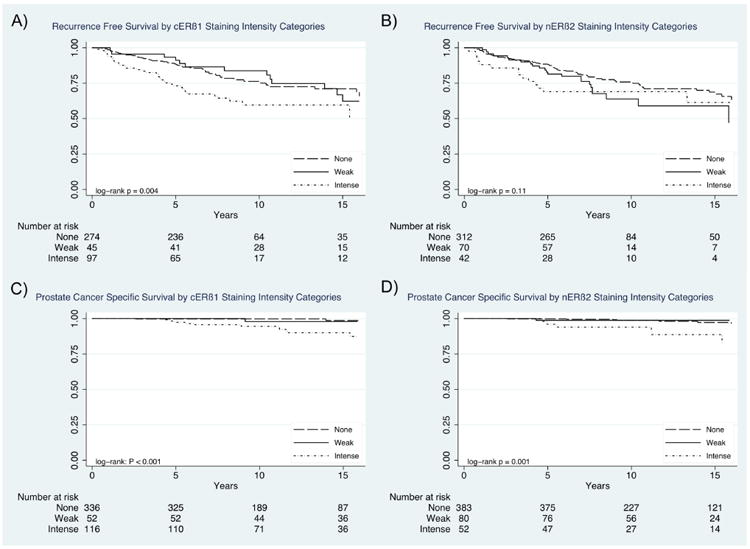
Prostate cancer recurrence free probability (A, B) and prostate cancer-specific survival probability (C, D) stratified by cERβ1 and nERβ2 staining intensity.
Table 3. Multivariable adjusted hazard ratios for prostate cancer recurrence and prostate cancer-specific mortality following radical prostatectomy by estrogen receptor subtype staining intensity.
| Recurrence Free Survival | Prostate Cancer-Specific Mortality$ | |||
|---|---|---|---|---|
|
|
||||
| *HR (95% CI) | p-value | *HR (95% CI) | p-value | |
| Nuclear ERβ2 | ||||
| No Staining | Referent | |||
| Weak Staining | 0.61 (0.07-5.16) | 0.65 | ||
| Intense Staining | 3.89 (1.12-13.42) | 0.03 | ||
|
| ||||
| Cytoplasmic ERβ1 | ||||
| No Staining | Referent | Referent | ||
| Weak Staining | 0.97 (0.53-1.80) | 0.93 | 1.11 (0.11-11.36) | 0.93 |
| Intense Staining | 1.72 (1.13-2.62) | 0.01 | 6.62 (1.75-24.95) | 0.01 |
All models adjusted for age, Gleason sum, pathologic stage and PSA at diagnosis; HR=hazard ratio, CI=confidence interval.
Values represent results from separate Cox proportional hazard models containing clinical factors* and the specified receptor.
Note: Nuclear ERβ2 staining was not significantly associated with recurrence free survival on co-variate analysis and as a result was not included in multivariable analysis.
PCa-specific death was observed in 13 (2.3%) men, resulting in a 3.2% estimated 15-year PCSM. Estimated 15-year PCSM probabilities were significantly different across cERβ1 staining strata at 9.7% vs. 2.1% vs. 1.4% (p=0.003) for intense vs. weak vs. no staining, respectively (Figure 3). Similarly,15-year PCSM probabilities were significantly different across nERβ2 staining strata at 11.3% for intense vs. 1.9% for weak vs. 2.7% for no staining, respectively (p=0.02). On unique multivariable analyses controlling for patient age, Gleason score, pathologic stage and diagnostic PSA, intense cERβ1 (HR 6.6, 95% CI 1.8 – 24.9, p=0.01) and intense nERβ2 (HR 3.9, 95% CI 1.1 – 13.4, p=0.03) staining were both associated with an increased risk of PCSM. We then evaluated the effect of cERβ1 and nERβ2 co-expression using the Kaplan-Meier method, demonstrating estimated 15-year PCSM of 16.4% vs. 4.3% vs. 0% vs. 2.0 % (p=0.001) for patients who expressed cERβ1 and nERβ2 vs. only cERβ1 vs. only nERβ2 vs. neither (Figure 4). ERβ5 and GPR30 staining distributions were not associated with RFS or PCSM.
Figure 4.
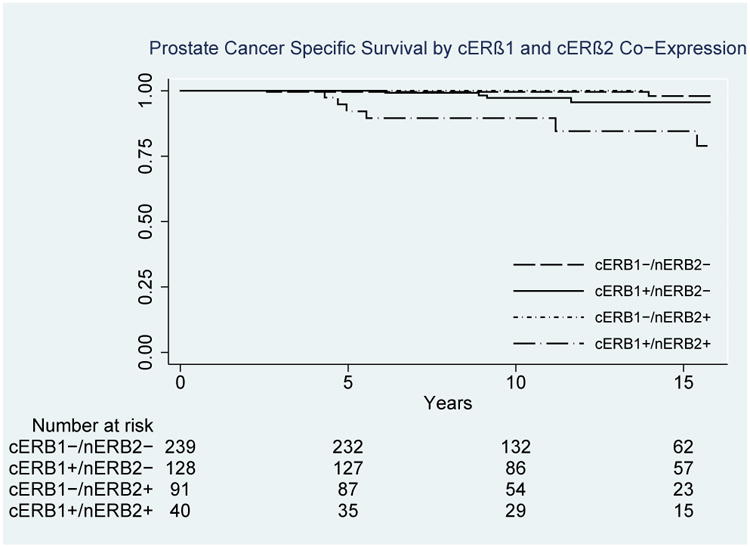
Prostate cancer-specific survival probability stratified by co-staining status of cERβ1 and nERβ2 staining.
Discussion
We evaluated the relationship between ER expression patterns and PCa outcomes in a population based cohort (n=566) of men with clinically localized disease undergoing RP with long-term follow-up. Intense expression of cERβ1 within tumors was independently associated with worse RFS (HR 1.7, 95% CI 1.1–2.6) and PCSM (HR 6.6, 95% CI 1.8-24.9) with significantly worse 5 year RFS (73.2% vs. 91.1% vs. 88.3% for intense vs. weak vs. none, respectively, p=0.004) and 15 year PCSM (9.7% vs. 2.1% and 1.4% for intense vs. weak vs. none, respectively, p=0.003). Similarly, intense staining of nERβ2 (HR 3.9, 95% CI 1.1-13.4) was independently associated with increased risk of PCSM with significantly worse 15 year PCSM (11.3% vs. 1.9% vs. 2.7% for intense vs. weak vs. no staining, respectively, p=0.02). Further, co-expression of cERβ1 and nERβ2 was associated with a significantly worse 15-year PCSM compared to patients expressing cERβ1 alone, nERβ2 alone, or neither. These data suggest that cERβ1 and nERβ2 may be useful prognostic biomarkers to identify men undergoing RP who are higher risk for adverse outcomes.
These findings contribute to the literature evaluating the role of ERβ in prostate carcinogenesis and prognosis. In particular, this study is the first to demonstrate a potential link between cERβ1 expression and adverse post-RP outcomes. These observations were not expected based on the hypothesized anti-proliferative effects of ERβ15 and a study by Leung et al, in which no associations between cERβ1 expression and PCa outcomes were observed using the same ERβ1 antibody to stain the tumors of 144 men undergoing RP.14 However, ERβ1 staining distributions were highly similar in both studies suggesting that the interpretation of ERβ1 staining was accurate and does not account for the differences observed between cERβ1 and clinical outcomes. There were, however, important differences between our study and the study by Leung et al. which may account for discrepancies in the relationship between ERβ1 and post-RP outcomes. First, we controlled for pathologic stage, which is known to impact recurrence and survival20,21 and was associated with cERβ1 staining intensity in our cohort. Second, we examined RFS and PCSM compared to PSA recurrence and post-RP metastases. Third, with longer follow-up and nearly quadruple the number of patients, our study had improved statistical power to detect relationships between ERβ subtypes and PCa-specific outcomes. Associations between cERβ1 staining and worse post-RP outcomes are consistent with data demonstrating expression of ERβ1(or WT ERβ) in the majority of nodal and boney metastases8,9 and PCa tumor expression of WT ERβ being associated with increased risk of recurrence following RP22 and worse overall survival in hormone naïve patients with metastatic disease11.
Our observations linking nERβ2 to poor PCa prognosis are consistent with previous studies. Specifically, the nearly 4-fold increased risk of PCSM among men with intense nERβ2 expression in this study is similar to the findings of Fujimura et. al. in which they identified elevated ERβ2 expression as a risk factor for PCSM in a cohort of 50 men.23 Additionally, while nERβ2 staining was not significantly associated with overall RFS (p=0.11) in our cohort, intense nERβ2 staining was significantly associated with 5-year RFS (69.1 % vs. 81.4% vs. 88.1% for intense vs. light vs. no staining) on Kaplan-Meir analysis, similar to the study by Leung et al in which high nERβ2 expression was independently associated with increased risk of biochemical recurrence and post-operative metastases.14 These clinical data are further supported by in vitro observations demonstrating increased invasiveness of PC3 cells expressing ERβ2.14 Additionally, ERβ2 expression has been associated with increased cellular proliferation and the expression of proliferation associated genes both in vitro and in mouse engrafts with up-regulation of mediators of boney metastases.24 Thus, biologic evidence supports increasingly aggressive cellular behavior with increasing ERβ2 expression in line with the observed poor outcomes following RP in patients with greater nERβ2 expression.
The impact of interactions between different ERβ isoforms on the biology of PCa is currently under investigation. However, evidence suggests that ERβ2 has no innate activity of its own and does not homodimerize, instead forming heterodimers with ERβ1 resulting in modulation of its activity.12,13 Further, ERβ2 acts as a transcriptional repressor of ERβ1 (thus inhibiting its usual anti-proliferative effects)25 suggesting that ERβ2 may therefore function as a dominant-negative regulator of ERβ1 via heterodimerization. As a result, one possible hypothesis to explain the observed association between intense cERβ1 staining and adverse post-RP outcomes is an interaction between ERβ1 and ERβ2 in the cytoplasm. As 99% of patients with ERβ2 staining had cERβ2, nearly all patients with cERβ1 were also cERβ2. Consequently, intense cERβ1 staining could identify those patients with the greatest degree of dominant negative heterodimerzation between cERβ1 and cERβ2, possibly preventing translocation of ERβ1 into the nucleus and thereby preventing the expected antiproliferative effects of ERβ1. Similar interactions between ERβ1 and ERβ2 could also account for the association observed between intense nERβ2 staining and increased PCSM, with nERβ2 forming dominant negative heterodimers with ERβ1 in the nucleus of tumor cells. As the current study cannot address this hypothesis, further studies are needed to evaluate the impact of ERβ1 and ERβ2 co-localization/interaction on ERβ signaling and subsequent downstream effects on PCa biology and patient outcomes. If such interactions were confirmed to be biologically important, selective ERβ isoform agonists/antagonists could potentially serve as new targeted agents in the management of PCa.
Limitations of this study include the potential for unmeasured confounding, however, our dataset includes clinical, pathological and epidemiological factors previously associated with PCa outcomes. Additionally, despite 566 patients in our cohort with relatively long median follow-up (>10 years) among survivors, only 3.2% experience PCSM, limiting the statistical power. Even with these limitations, our results suggest that patients whose tumors express increased cERβ1 and nERβ2 are at particularly high-risk and may warrant closer surveillance following RP.
Conclusion
Men whose tumors highly express cERβ1 and/or nERβ2 may have increased risk of adverse PCa-specific outcomes following RP. If confirmed, these findings suggest that evaluation of ERβ1 and ERβ2 expression at the time of RP could provide important prognostic information and inform post-RP surveillance strategies.
Acknowledgments
Funding Sources: This work was supported by grants R01-CA056678, R01-CA092579, R03-CA137799, and P50-CA097186 from the National Cancer Institute, with additional support from the Fred Hutchinson Cancer Research Center, the Prostate Cancer Foundation, and the Institute for Prostate Cancer Research.
Abbreviations
- ER
Estrogen Receptor
- FFPE
Formalin Fixed Paraffin Embedded
- PCa
Prostate Cancer
- PCSM
Prostate Cancer Specific Mortality
- RFS
Recurrence Free Survival
- RP
Radical Prostatectomy
- TMA
Tissue Microarray
- WT
Wild Type
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Rohrmann S, Nelson WG, Rifai N, et al. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab. 2007;92(7):2519–2525. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- 3.Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 4.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pravettoni A, Mornati O, Martini PG, et al. Estrogen receptor beta (ERbeta) and inhibition of prostate cancer cell proliferation: studies on the possible mechanism of action in DU145 cells. Mol Cell Endocrinol. 2007;263(1-2):46–54. doi: 10.1016/j.mce.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Krege JH, Hodgin JB, Couse JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95(26):15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weihua Z, Makela S, Andersson LC, et al. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci U S A. 2001;98(11):6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leav I, Lau KM, Adams JY, et al. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159(1):79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fixemer T, Remberger K, Bonkhoff H. Differential expression of the estrogen receptor beta (ERbeta) in human prostate tissue, premalignant changes, and in primary, metastatic, and recurrent prostatic adenocarcinoma. Prostate. 2003;54(2):79–87. doi: 10.1002/pros.10171. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X, Leav I, Leung YK, et al. Dynamic regulation of estrogen receptor-beta expression by DNA methylation during prostate cancer development and metastasis. Am J Pathol. 2004;164(6):2003–2012. doi: 10.1016/s0002-9440(10)63760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zellweger T, Stürm S, Rey S, et al. Estrogen receptor β expression and androgen receptor phosphorylation correlate with a poor clinical outcome in hormone-naive prostate cancer and are elevated in castration-resistant disease. Endocr Relat Cancer. 2013;20(3):403–413. doi: 10.1530/ERC-12-0402. [DOI] [PubMed] [Google Scholar]
- 12.Moore JT, McKee DD, Slentz-Kesler K, et al. Cloning and characterization of human estrogen receptor beta isoforms. Biochem Biophys Res Commun. 1998;247(1):75–78. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- 13.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc Natl Acad Sci U S A. 2006;103(35):13162–13167. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung YK, Lam HM, Wu S, et al. Estrogen receptor beta2 and beta5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocr Relat Cancer. 2010;17(3):675–689. doi: 10.1677/ERC-09-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan QK, Lam HM, Ng CF, et al. Activation of GPR30 inhibits the growth of prostate cancer cells through sustained activation of Erk1/2, c-jun/c-fos-dependent upregulation of p21, and induction of G(2) cell-cycle arrest. Cell Death Differ. 2010;17(9):1511–1523. doi: 10.1038/cdd.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanford JL, Wicklund KG, McKnight B, Daling JR, Brawer MK. Vasectomy and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1999;8(10):881–886. [PubMed] [Google Scholar]
- 17.Agalliu I, Salinas CA, Hansten PD, Ostrander EA, Stanford JL. Statin use and risk of prostate cancer: results from a population-based epidemiologic study. Am J Epidemiol. 2008;168(3):250–260. doi: 10.1093/aje/kwn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam HM, Suresh Babu CV, Wang J, et al. Phosphorylation of human estrogen receptor-beta at serine 105 inhibits breast cancer cell migration and invasion. Mol Cell Endocrinol. 2012;358(1):27–35. doi: 10.1016/j.mce.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FitzGerald LM, Zhang X, Kolb S, et al. Investigation of the relationship between prostate cancer and MSMB and NCOA4 genetic variants and protein expression. Hum Mutat. 2013;34(1):149–156. doi: 10.1002/humu.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 21.Lerner SE, Blute ML, Bergstralh EJ, Bostwick DG, Eickholt JT, Zincke H. Analysis of risk factors for progression in patients with pathologically confined prostate cancers after radical retropubic prostatectomy. J Urol. 1996;156(1):137–143. [PubMed] [Google Scholar]
- 22.Horvath LG, Henshall SM, Lee CS, et al. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res. 2001;61(14):5331–5335. [PubMed] [Google Scholar]
- 23.Fujimura T, Takahashi S, Urano T, et al. Differential expression of estrogen receptor beta (ERbeta) and its C-terminal truncated splice variant ERbetacx as prognostic predictors in human prostatic cancer. Biochem Biophys Res Commun. 2001;289(3):692–699. doi: 10.1006/bbrc.2001.6038. [DOI] [PubMed] [Google Scholar]
- 24.Dey P, Jonsson P, Hartman J, Williams C, Ström A, Gustafsson J. Estrogen receptors β1 and β2 have opposing roles in regulating proliferation and bone metastasis genes in the prostate cancer cell line PC3. Mol Endocrinol. 2012;26(12):1991–2003. doi: 10.1210/me.2012.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cotrim CZ, Fabris V, Doria ML, et al. Estrogen receptor beta growth-inhibitory effects are repressed through activation of MAPK and PI3K signalling in mammary epithelial and breast cancer cells. Oncogene. 2013;32(19):2390–2402. doi: 10.1038/onc.2012.261. [DOI] [PubMed] [Google Scholar]


