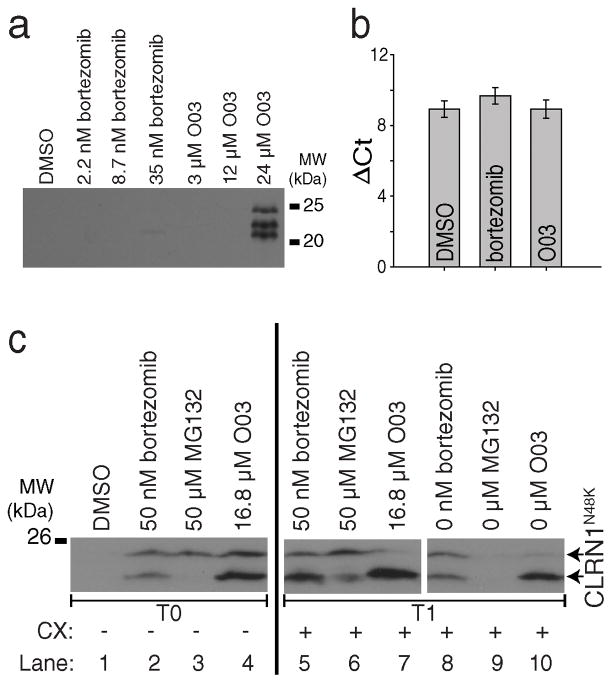
Figure 3. The lead compound O03 stabilizes CLRN1N48K by a post-translational mechanism.
(a) Mouse CLRN1N48K expressed in NIH/3T3 cells was stabilized by O03 treatment. (b) Quantitative RT-PCR of HEK293 cells stably expressing human CLRN1N48K. CLRN1 mRNA levels were not affected by either bortezomib or O03 treatment (O03 vs. DMSO, P = 0.9995, two-sided t-test, n=4, means ± SDs). (c) Protein synthesis is not required for the effect of O03. Cells treated with proteasome inhibitors bortezomib and MG132 showed increased levels of CLRN1N48K as did O03 treatment when compared to DMSO (lanes 1–4, T0). CLRN1N48K levels remained similar after treatment for an additional 6 h in the presence of 100 μM cycloheximide (CX) (lanes 5–7, T1), indicating that O03 stabilized CLRN1N48K in the absence of protein synthesis. To confirm that CLRN1N48K can be effectively degraded in the absence of protein synthesis, cells were treated with bortezomib, MG132, or O03 for 6 h. Compounds then were washed out, and cells were further incubated for 6 h in the presence of CX (lanes 8 – 10, T1). CLRN1N48K was undetectable in cells after removing the reversible proteasome inhibitor MG132 (compare lanes 3 and 9). Effects of O03 and bortezomib persisted 6 h after the washout. Note: Multiple immunopositive bands were detected by the HA-antibody which recognizes the Ct-tail of CLRN1N48K (a and c). Based on the analysis of the primary structure39, CLRN1 contains a few signal peptide cleavage sites; incomplete cleavage leads to a few differently sized bands. See also Supplementary Figure 13.