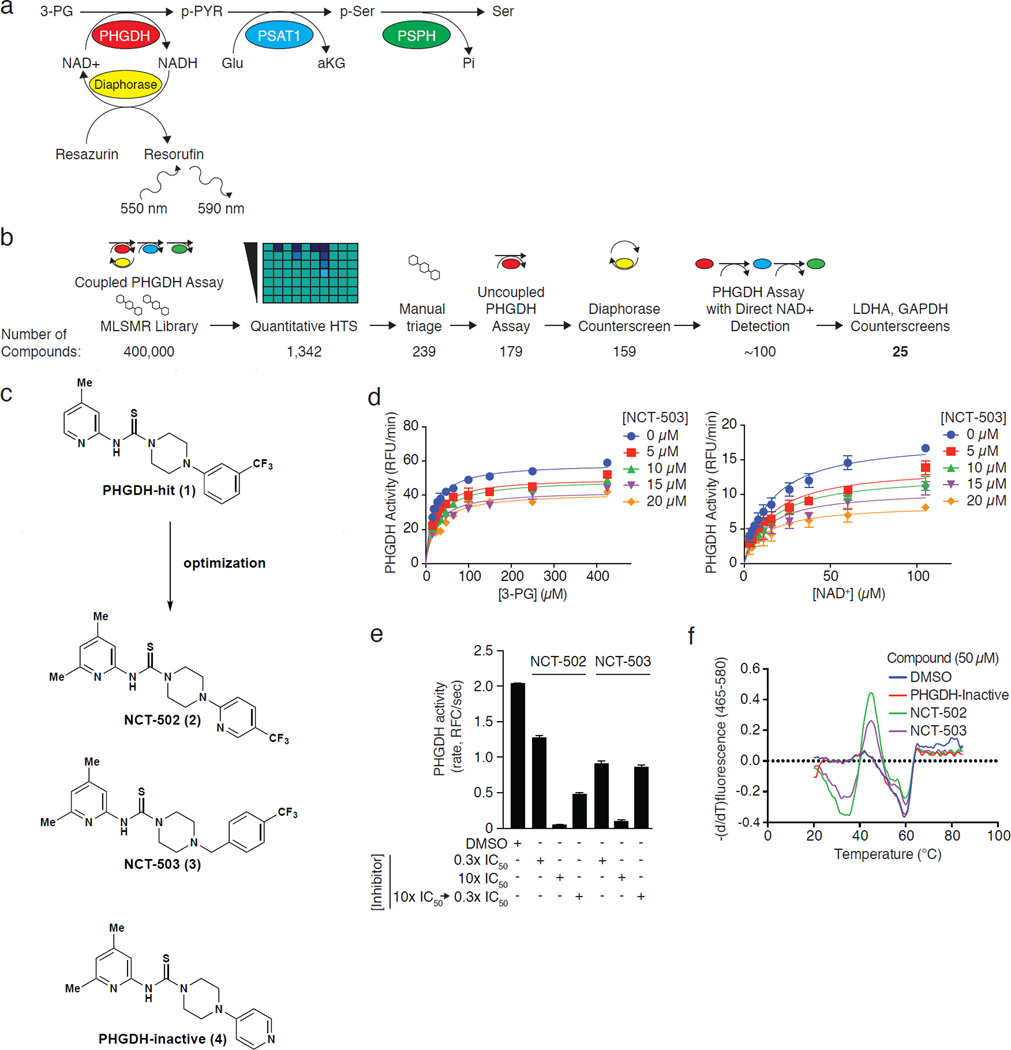
Figure 1. Identification and characterization of small molecule PHGDH inhibitors.
a, Coupled PHGDH assay with diaphorase/resazurin readout used for the primary screen. b, Screening pipeline for PHGDH inhibitors. Following HTS, manual triage selected synthetically tractable compounds and eliminated promiscuous inhibitors. Remaining compounds were confirmed and counterscreened to eliminate false positives and pan-dehydrogenase inhibitors. The number of compounds remaining is listed beneath each step. c, Piperazine-1-carbothioamide PHGDH inhibitors. PHGDH-hit (1) was the initial hit in the screen; NCT-502 (2) was a derivative with improved potency, and NCT-503 (3) has improved solubility and in vivo characteristics. The structurally related inactive compound (PHGDH-inactive; 4) had no activity against PHGDH and served as a negative control. d, NCT-503 exhibits noncompetitive inhibition with respect to both 3-PG and NAD+. Data are average of three experiments and error bars represent standard deviations. e, Dilution data demonstrating in vitro reversibility of NCT-502 and NCT-503. Data are average of 96 experiments and error bars represent standard deviations. f, Melting temperature curves demonstrating NCT-502 and NCT-503-induced destabilization of PHGDH. Curves are representative of 3 experiments.