Fig. 1.
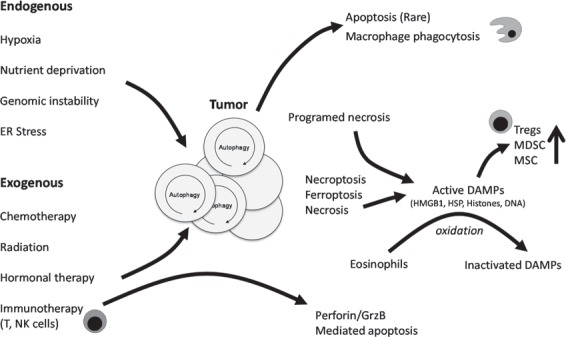
The tumor microenvironment is disordered and characterized by necrosis, redox stress, and DAMPs. Autophagy within the tumor can be promoted by several endogenous (hypoxia, nutrient deprivation, genomic instability, ER stress) and exogenous (chemotherapy, radiation, hormonal therapy) stressors. Most of the current treatment strategies for cancer promote (DAMP molecule release (HMGB1, HSP, histones, DNA etc.) following therapy-induced unscheduled tumor death (often by necroptosis and necrosis). Solely apoptotic cell death in the tumor, intrinsic and driven by p53 pathways, extrinsic, promoted by tissue macrophages and other immune cells expressing TNF family members, is a rare finding. Immunotherapy promoting NK and Teff function can also lead to cytolytic (Perforin/GrzB) mediated apoptosis in the tumor. Therapy promoted DAMP release leads to recruitment of myeloid cells (monocytes, DCs, and granulocytes), as well as MSCs. They belong to the early immigrants in response to unscheduled cell death, initiating and modulating the subsequent inflammatory response. MSCs as well as DCs respond to DAMPs by promoting an immunosuppressive milieu, while eosinophils induce oxidative conditions limiting the biologic activity of DAMPs over time and distance. Tregs are strongly affected by pattern recognition receptor signaling in the tumor microenvironment and limit immune reactivity coordinately with myeloid-derived suppressor cells.
