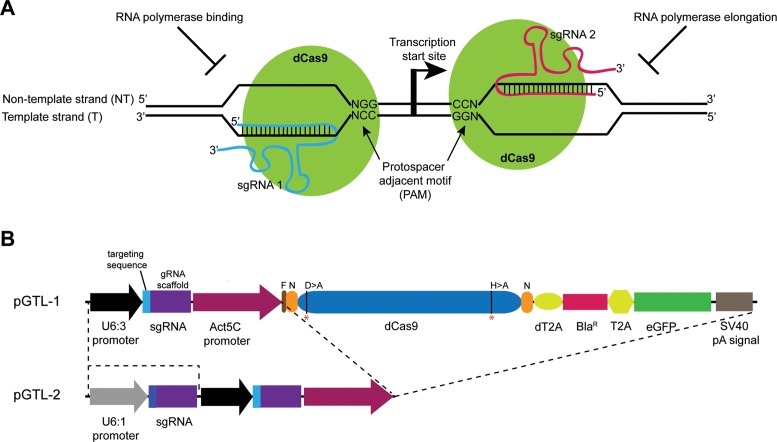
Figure 1.
CRISPRi in Drosophila. (A) Diagram showing the CRISPR interference (CRISPRi) system. To repress transcription, the catalytically inactive Cas9 protein (dCas9, green) is targeted either to the template or non-template DNA strand based on the targeting sequence of the sgRNA and an adjacent PAM sequence. Binding of the dCas9:sgRNA complex upstream of the transcription start site interferes with transcription initiation by preventing recruitment of the RNA polymerase while its assembly at a downstream site prevents transcription elongation. (B) Schematic representation of the transfection vectors pGTL-1 and pGTL-2. In pGTL-1, a single guide RNA with a 20 nt targeting sequence and the dCas9 protein are coexpressed under Drosophila constitutive promoters U6:3 and Actin5C, respectively. The dCas9 is separated from the blasticidin resistance gene (BlaR) and eGFP by self-cleaving T2A peptides (dT2A and T2A). The guide RNA (gRNA) scaffold contains the U6 transcription terminator sequence. The pGTL-2 vector contains an additional sgRNA scaffold under the U6:1 promoter, thus allowing production of two sgRNAs simultaneously with the dCas9 protein. The mutated amino acid residues (D10>A and H841>A) in dCas9 are marked with red asterisks (*). N = NLS sequence, F = FLAG epitope, pA = polyadenylation.