Figure 12.
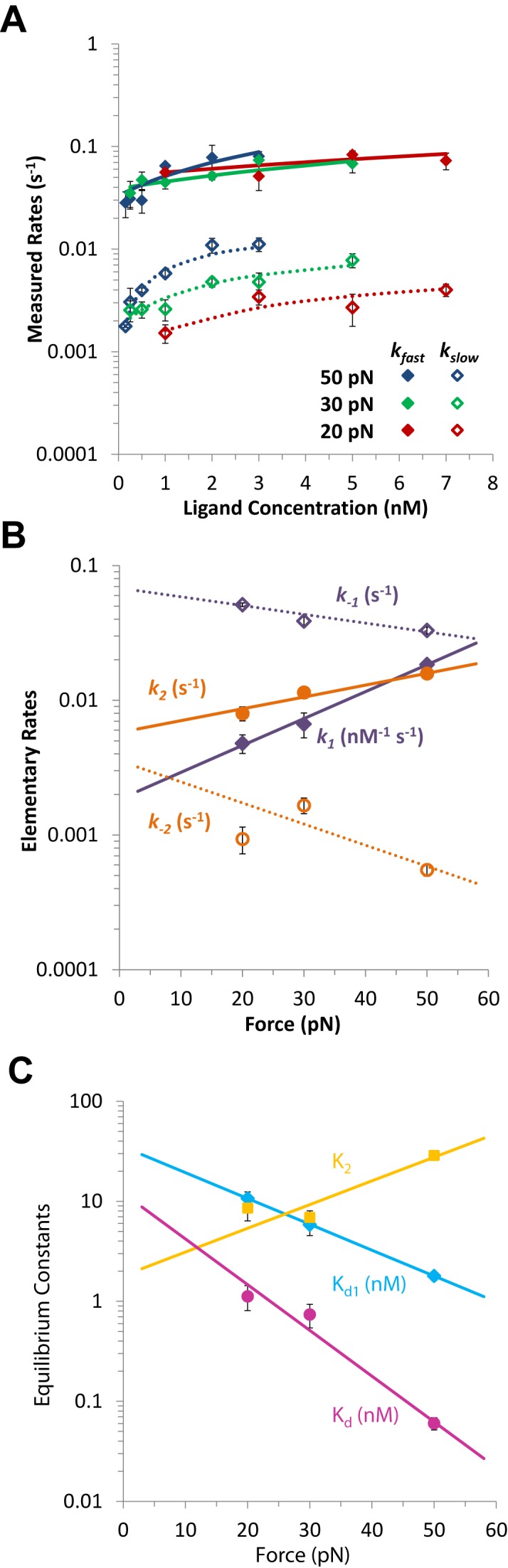
Kinetics of Δ,Δ-Pc binding to dsDNA, from (44). (A) Measured fast and slow rates versus concentration of Δ,Δ-Pc at three forces: 20, 30 and 50 pN (red, green and blue). Data points are rates kf and ks obtained by fitting the length versus time for the Δ,Δ-Pc/DNA complex to Equation ((18)). Lines are the results of fits to Equation ((17)) (solid line fits for kf and dotted lines fits for ks) which determine the elementary rates of the two-step reaction. (B) Fitted values of elementary rates for two-step intercalation, giving the forward rates k1 and k2 (solid purple and orange symbols) and reverse rates k-1 and k-2 (open purple and orange symbols). Lines represent fits to Equation ((19)), and give the force-independent elementary rates and transition distances. Fitted parameters are shown in Table 3. (C) Force-dependent binding constants for each step Kd1 (cyan) and K2 (gold) and for overall binding Kd (magenta), determined from the elementary rates. Lines denote fits to the exponential force dependence, which give the zero-force binding constants and equilibrium length changes.
