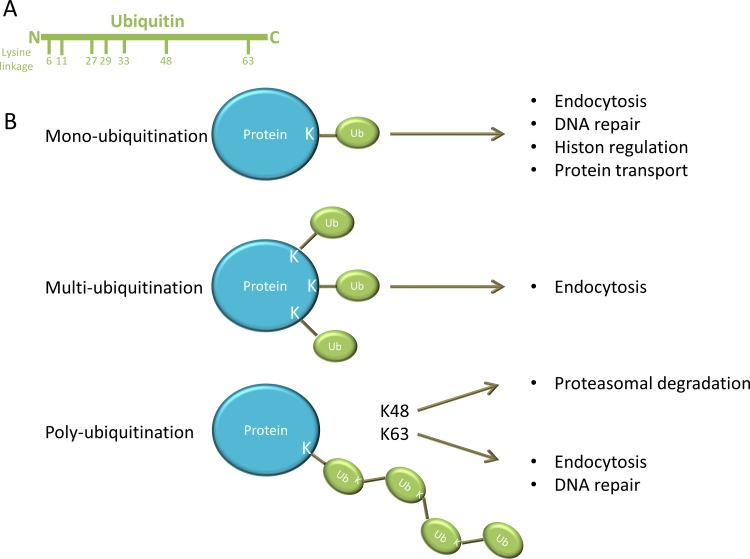
Figure 1. different forms of ubiquitin modification.
A. Ubiquitin is composed of 76 amino acids and contains seven lysine residues on which other ubiquitin molecules can be conjugated. B. A single ubiquitin is linked to a lysine (K) residue on the protein during mono-ubiquitination. Mono-ubiquitination is involved in endocytosis, DNA repair, histon regulation and protein transport. Multi-ubiquitination is obtained when multiple single ubiquitins are linked to different lysine residues on the protein and is implicated in endocytosis. However poly-ubiquitination is obtained when a poly-ubiquitin chain is linked to the protein. Poly-ubiquitin chains formed on lysine 48 (K48) of ubiquitin have a well-known role in targeted protein degradation by the 26S proteasome, whereas poly-ubiquitin chains formed through lysine 63 (K63) are involved in DNA repair and endocytosis.