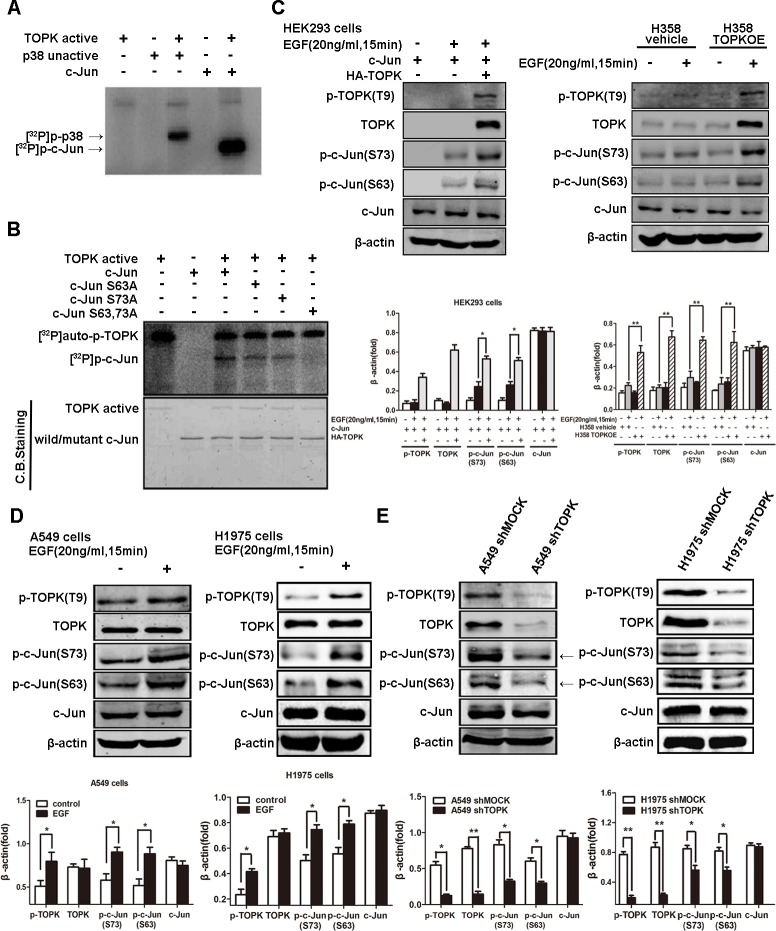
Figure 5. TOPK phosphorylates c-Jun at serine 63 and serine 73.
A. Active TOPK phosphorylates full-length c-Jun in vitro in the presence of [γ-32P] ATP. The incorporation of 32P was visualized by autoradiography. Inactive p38 protein was used as a positive control. B. Active TOPK phosphorylation of wild-type c-Jun, c-Jun(S63A), c-Jun(S73A) and c-Jun (S63A&S73A) was visualized by autoradiography. Equal protein loading was visualized by Coomassie Blue (CB) staining. C. Ectopic expression of TOPK increases c-Jun phosphorylation at the Ser63 and Ser73 sites. Cells were transfected with pcDNA3.1A-HA-TOPK and pcDNA4-His-c-Jun and cultured for 24 h. After starvation in DMEM supplemented with 0.1% FBS for 24 h, cells were stimulated with EGF (20 ng/mL) and harvested 15 min later. Whole cell lysates were then analyzed by Western blotting. A representative blot was presented. All protein levels were measured with densitometry and normalized to β-actin. Each bar represents the mean±SD from three experiments.*p < 0.05, **p < 0.01. D. EGF induces TOPK activation and c-Jun phosphorylation at Ser63 and Ser73 in gefitinib-resistant lung cancer cells. After starvation in RPMI 1640 supplemented with 0.1% FBS for 24 h, cells were stimulated with EGF (20 ng/mL) and harvested 15 min later. Whole cell lysates were then analyzed by Western blotting. All protein levels were measured with densitometry and normalized to β-actin. Each bar represents the mean±SD from three experiments.*p < 0.05, **p < 0.01. E. Knockdown of TOPK decreases the phosphorylation level of c-Jun. The whole cell lysates were analyzed by Western blotting. A representative blot was presented. All protein levels were measured with densitometry and normalized to β-actin. Each bar represents the mean±SD from three experiments.*p < 0.05, **p < 0.01.