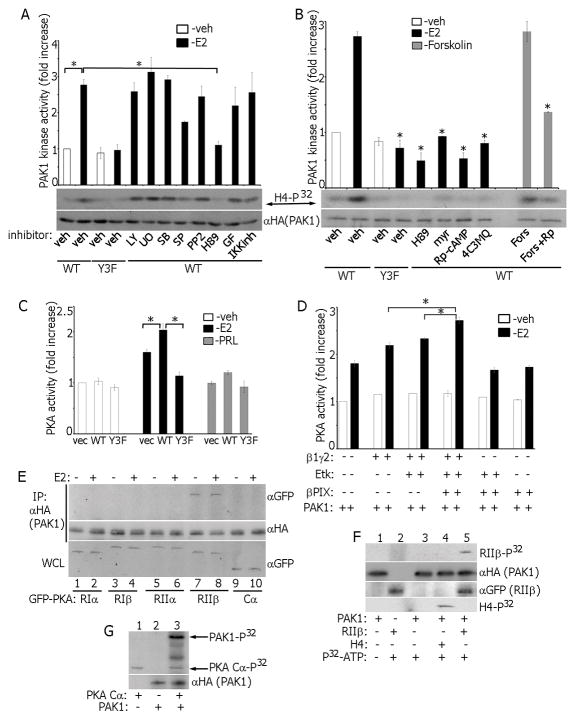
Figure 6.
Reciprocal regulation of PAK1 and PKA in E2 signaling. (A) Cells were treated with inhibitors: UO126, SB203580, SP600125, LY294002, IKK inhibitor, GF109203X and H89, then with E2 and PAK1 activation was assessed in the in vitro kinase assay. (B) PAK1 activation was assessed as in A in cells treated with indicated different PKA inhibitors. *, P < 0.05 compared with cells expressing PAK1 WT and treated with veh+E2. (C) Cell lysates were assessed for PKA activity in the in vitro kinase assay. (D) Combinations of β1γ2 subunits, Etk and βPIX were overexpressed in MCF-7 PAK1 WT cells. E2-dependent PKA activation was assessed in the in vitro kinase assay. (E) mEGFP-tagged cDNAs encoding different PKA subunits (RIα, RIβ, RIIα, RIIβ and Cα) were overexpressed in MCF-7 PAK1 WT clone and treated with/without E2. HA-PAK1 was IP’s with αHA and immunoblotted. (F) In vitro translated PAK1 was incubated with IP’d PKA RIIβ in the in vitro kinase assay. H4 histone was used as a PAK1 substrate (lane 4). (G) In vitro translated PAK1 was incubated with recombinant active PKA Cα (catalytic subunit) in the in vitro kinase assay. Incorporation of P32 in PAK1 (upper arrow) and in PKA Cα (low arrows) is indicated.