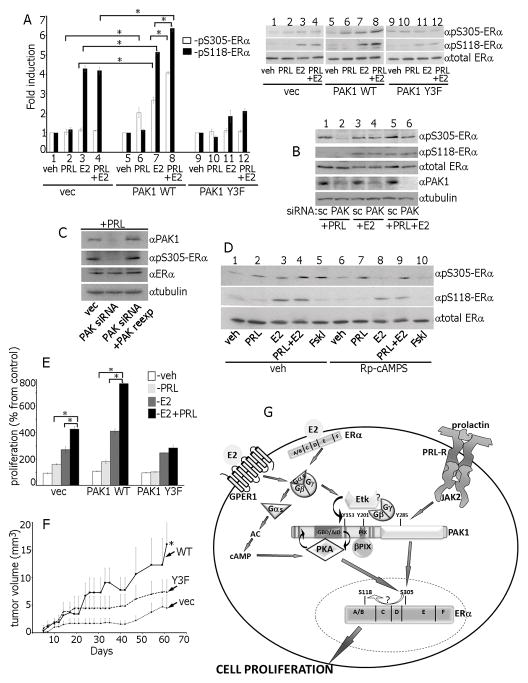
Figure 7.
Ser305-ERα is phosphorylated by PKA in response to E2 and by PAK1 in response to PRL. (A) Control, PAK1 WT or PAK1 Y3F clones of MCF-7 cells were treated with veh, E2, PRL or PRL+E2 and WCL were immunoblotted. (B) Cells were transfected with control (scr) or PAK1 siRNA, treated with PRL, E2 or PRL+E2 and WCL were immunoblotted. (C) In siRNA rescue experiments, 24h after PAK1 siRNA transfection, cells were transfected with control or PAK1 WT, treated with PRL and WCL were immunoblotted. (D) Cells were incubated with either veh or Rp-cAMP, treated with veh, PRL, E2, PRL+E2 or Forskolin. (E) MCF-7 overexpressing vec, PAK1 WT or PAK1 Y3F were incubated with veh, PRL, E2 or PRL+E2 and assessed for cell proliferation as in Fig.1. (F) pTyr-PAK1 accelerates tumor growth in vivo. MCF-7 clones were injected into the mammary pad of female NSG mice. Mice were treated with PRL+E2. *, p<0.05 compared with control (vec) mice, n=8. (G) Proposed mechanisms for the role of PAK1 in synergetic effects of PRL and E2 on ERα activation. E2 activates membrane-localized ERα and GPER1 leading to G-protein and Etk kinase activation. E2-activated Etk binds to and directly phosphorylates PAK1 on Tyr153. Gβγ subunits also bind to pTyr-PAK1, facilitating recruitment and activation of βPIX, which in turn binds and further activates PAK1 and establishes a self-amplifying cascade. Gαs subunit activates PKA, which binds to PAK1 via PKA-RIIβ while PKA-Cα directly phosphorylates PAK1. Gβγ/Etk/pTyr-PAK1/βPIX complex reciprocally activates PKA in response to E2 and activated PKA phosphorylates Ser305-ERα leading to enhanced S118-ERα phosphorylation. PRL-activated JAK2 phosphorylates and activates PAK1 which also activates ERα by phosphorylation of S305-ERα. Thus, in response to E2+PRL, pS305-ERα is phosphorylated by both PAK1 and PKA that leads to enhanced cell proliferation.