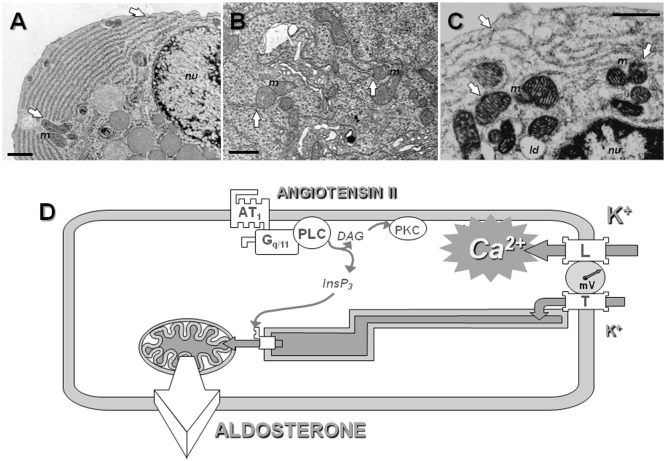
Figure 3.
The concept of intracellular calcium pipeline: a model for explaining the selective transport of calcium from the T channels into the mitochondria. Electron microscopy reveals the presence of close apposition in many places of the endoplasmic reticulum (ER) with the plasma membrane or the mitochondria (white arrows), within various cell types, including rat parotid cells (A), rat spinal cord neurons (B), or bovine adrenal glomerulosa cells (C). Scale = 1 μm; m indicates mitochondria, nu nucleus and ld lipid droplets. See Ref. (112) for additional information. (D). A hypothetical model for the cellular transport of calcium into mitochondria. At the pipeline filling site, T-type calcium channels and, to a lesser extent, L-type channels are activated upon cell depolarization by potassium or angiotensin II. Several experimental data suggest that calcium entering the cell through T-type channels could be selectively pumped into the lumen of the ER, while calcium entering through L-type channels would be poured into the cytosol. At the pipeline delivery site, InsP3 receptors are maintained in proximity of the mitochondria within “quasi synaptic” structures. Calcium released upon activation of the InsP3 receptors, due to calcium overloading of the ER and/or to InsP3 production by AT1 receptor-activated PLC, is rapidly internalized into the very negatively charged matrix, through the mitochondrial inner membrane calcium uniporter. Intramitochondrial calcium elevation then stimulates limiting steps of aldosterone biosynthesis. AT1, angiotensin II receptor, type 1; Gq/11, heterotrimeric G protein of the q/11 family; PLC, phospholipase C β; PKC, protein kinase C; InsP3, inositol 1,4,5-trisphosphate.