Abstract
We decipher the resistome of Chryseobacterium indologenes MARS15, an emerging multidrug-resistant clinical strain, using the whole genome sequencing strategy. The bacterium was isolated from the sputum of a hospitalized patient with cystic fibrosis in the Timone Hospital in Marseille, France. Genome sequencing was done with Illumina MiSeq using a paired-end strategy. The in silico analysis was done by RAST, the resistome by the ARG-ANNOT database and detection of polyketide synthase (PKS) by ANTISMAH. The genome size of C. indologenes MARS15 is 4 972 580 bp with 36.4% GC content. This multidrug-resistant bacterium was resistant to all β-lactams, including imipenem, and also to colistin. The resistome of C. indologenes MARS15 includes Ambler class A and B β-lactams encoding blaCIA and blaIND-2 genes and MBL (metallo-β-lactamase) genes, the CAT (chloramphenicol acetyltransferase) gene and the multidrug efflux pump AcrB. Specific features include the presence of an urease operon, an intact prophage and a carotenoid biosynthesis pathway. Interestingly, we report for the first time in C. indologenes a PKS cluster that might be responsible for secondary metabolite biosynthesis, similar to erythromycin. The whole genome sequence analysis provides insight into the resistome and the discovery of new details, such as the PKS cluster.
Keywords: Carotenoid, Chryseobacterium indologenes, microbial genomics, nonribosomal polyketide synthase, polyketide synthase, resistome, urease
Introduction
The genus Chryseobacterium (family Flavobacteriaceae, phylum Bacteroidetes) [1] is a group of Gram-negative, nonfermenting, catalase-positive, oxidase-positive and indole-positive aerobic bacilli [2]. Chryseobacterium strains have been isolated from a variety of environments, including composted diseased fish, soil and plant rhizospheres [3].
C. indologenes is not normally present in the human microflora, although it is widely distributed in nature [4]. Chryseobacterium indologenes can be found in water systems despite chlorine treatment and on the wet surface of sink basins, taps, medical tools and other equipment [5], creating a potential reservoir for infection in hospital environments.
These primarily opportunistic pathogens infect mainly newborns [6] and patients with respiratory disease or immunosuppression [2]; patients with long-term indwelling devices may become colonized with this bacterium after contact with contaminated medical devices such as feeding tubes, intravascular catheters and endotracheal tubes [4].
The SENTRY Program [6], using results from over 119 sentinel hospitals and laboratories in North America, Latin America, Europe and the Asia-Pacific region in the initial 5 years of the program (1997 to 2001), revealed that Chryseobacterium species constitute only 0.03% of all bacterial isolates. All strains came from hospitalized patients. C. meningosepticum was the most frequently isolated microorganism, followed by C. indologenes. The lower respiratory tract (52%) and blood (46%) were the major sites where the microorganism was isolated [5].
The first clinical sample of C. indologenes was isolated in 1993 from the tracheal aspirate of a patient with ventilator-associated pneumonia [7].
C. indologenes can cause various types of infections [2], including keratitis, bacteraemia [2], pneumonia, cellulitis and artificial shunt infection in Taiwan [8], [9], [10], [11], Australia [12], Europe [13], [14], [15] and the United States [16].
There is no reference standard or guideline for the therapeutic management of C. indologenes infection despite increasing evidence of nosocomial-associated infections. An increased resistance rate to previously potent antibiotics suggests that a resistant pattern of C. indologenes may evolve over time and may vary according to different trends in antibiotic usage [17].
This bacterium is multiresistant to several antimicrobial drugs, particularly colistin, which is widely used for treatment of P. aeruginosa colonization in patients with cystic fibrosis (CF) [18], while it is usually susceptible to trimethoprim–sulfamethoxazole and piperacillin–tazobactam.
The goal of this study was to decipher the resistome and genome properties of clinical bacteria in the lung of CF patients using whole genome sequencing (WGS).
Materials and Methods
Growth condition and identification
C. indologenes was isolated on Cepacia medium (Becton Dickinson (BD), San Diego, CA, USA) from the sputum sample of a 15 years old CF girl regularly treated with aerosolized colistin for chronic Pseudomonas aeruginosa exacerbations.
Presumptive identification was done by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS; Microflex, Bruker Daltonics, Leipzig, Germany) using the flex control software (Bruker Daltonics) as previously described [19]. Growth was also tested using different media, including trypticase soy agar (TSA) (bioMérieux, Marcy-l’Étoile, France), Columbia agar with 5% sheep's blood (bioMérieux), selective chromogenic medium for the screening of extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae (bioMérieux) and different salt concentrations (2.5%, 5%, 7.5%, 10%, 15%). Electron microscopy was performed on a TechnaiG2 Cryo (FEI Company, Limeil-Brevannes, France) at an operating voltage of 200 keV. The biochemical test was performed by API20NE (bioMérieux).
Antibiotic susceptibility testing
Antibiotic susceptibility testing was done using the disk diffusion method on Mueller-Hinton agar medium (bioMérieux). The results were interpreted using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines [20], and minimum inhibitory concentrations (MICs) were determined by the Etest method (bioMérieux). Screening for metallo-β-lactamase activity was performed using modified Carba NP (MCNP) [21].
Genome sequencing
Genome sequencing of C. indologenes MARS15 was done by Illumina MiSeq (Illumina, San Diego, CA, USA) using the paired-end strategy and producing 2 × 250 bp (average read length). The assembly was performed using the SPAdes assembler, using different K values equal to 21, 33, 55, 77, 99 and 127, automatically chosen for this length read.
Genome annotation
The annotation was performed by Rapid Annotation using the Subsytem Technology (RAST) bioserver [22]. Gene Ontology (GO) in terms of Biological Process and Molecular Functions and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were considered. Detection of plasmid was performed by PlasmidFinder [23]. The Antibiotic Resistance Gene ANNotation (ARG-ANNOT database) was used to improve the annotation of the antibiotic resistance gene [24]. The intergenomic distances between genome sequences were determined from fully or partially sequenced genomes using average nucleotide identity and the percentage of conserved DNA (minimum identity, 70%) [25]. The genome of C. indologenes MARS15 was compared to the complete genome of Chryseobacterium sp. stRB126 (accession no. NZ_AP014624) and to the related genomes C. indologenes NBRC 14944 (accession no. NZ_BAVL00000000.1) and C. indologenes J31 (accession no. NZ_LAZY00000000.1). The Mauve alignment tool (version 2.3.1) was used for multiple genomic sequence alignment. RNAs were found by using RNAmmer [26]. PHAST (PHAge search Tool) was used to identify phage sequences [27]. The exhaustive bacteriocin database available in our laboratories (Bacteriocins from the URMITE database; http://drissifatima.wix.com/bacteriocins) was performed by collecting all currently available sequences from the databases and from the National Center for Biotechnology Information. Protein sequences from this database allowed putative bacteriocins from human gut microbiota to be identified using the BLASTp methodology [28].
Analyses for the presence of polyketide synthase (PKS) and nonribosomal peptide synthase (NRPS) were performed, discriminating the gene with large size using a database realized in our laboratory; predicted proteins were compared against the nonredundant (nr) GenBank database using BLASTp and analysed using the ANTibiotics and Secondary Metabolite Analysis Shell [29].
Results
Phenotypic properties
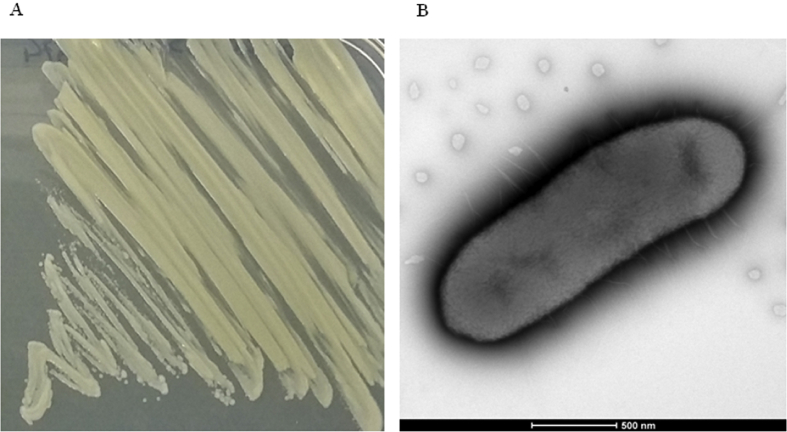
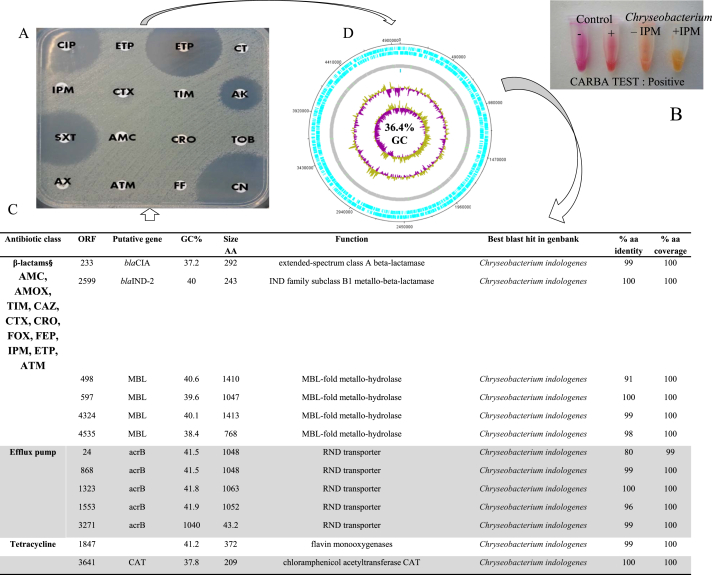
C. indologenes MARS15 (http://www.mediterranee-infection.com/arkotheque/client/ihumed/_depot_arko/articles/658/catalogue-csur-27-10-2015_doc.pdf) was isolated in March 2013 from the sputum sample of a 15-year-old girl with CF on Cepacia medium. The isolate appearance had a pigmented pale yellow colour (Fig. 1(A)). After 24 hours, the colonies were correctly identified by MALDI-TOF MS as C. indologenes, with a good score (>2.0). The isolate was able to grow on different culture media such as TSA and COS media but did not grow on ESBL medium. Moreover, the growth exhibited a high tolerance for sodium chloride at concentrations up to 5% after 32 hours of incubation. C. indologenes MARS15 is a Gram-negative, motile (via gliding) bacillus bacterium. The motility test was positive, and electron microscopy revealed the presence of a uniform distribution of peritrichous flagella around the cell wall (Fig. 1(B)). Phenotypically, C. indologenes MARS15 was resistant to amoxicillin (MIC >256 μg/mL), amoxicillin–clavulanic acid, ticarcillin–clavulanic acid, second-generation (cefoxitin) and third-generation cephalosporins (ceftazidime (MIC >6 μg/mL), ceftriaxone, imipenem (MIC >32 μg/mL), ertapenem, aminoglycoside (tobramycin), colistin (MIC >256 μg/mL), erythromycin (>4 μg/mL) and chloramphenicol, but was susceptible to gentamicin, amikacin, ciprofloxacin, trimethoprim–sulfamethoxazole and rifampicin (Fig. 2(A)). The modified Carba NP test method for carbapenemase-producing detection was positive (Fig. 2(B)).
Fig. 1.
(A) Chryseobacterium indologenes MARS15 isolated on Mueller-Hinton agar. (B) Electron microscopic image of C. indologenes MARS15 using TechnaiG2 Cryo at operating voltage of 200 keV.
Fig. 2.
From genotype to phenotype of Chryseobacterium indologenes MARS15. (A) Antibiotic sensitivity testing. AK, amikacin; AMC, amoxicillin–clavulanate acid; ATM, aztreonam; AX, amoxicillin; CIP, ciprofloxacin; CN, gentamicin; CRO, ceftriaxone; CT, colistin; CTX, cefotaxime; ETP, ertapenem; FOX, cefoxitin; IPM, imipenem; SXT, trimethoprim–sulfamethoxazole; TIC, ticarcillin; TIM, ticarcillin–clavulanate acid; TOB, tobramycin. (B) Results of modified Carba NP test; colour change of sample revealed presence of lactamases. (C) List of antibiotic-resistant genes. (D) Circular map of chromosome. From outside to center: genes on forward and reverse strand rRNA and tRNA (green), GC content and GC skew.
Genome features
The genome size of C. indologenes MARS15 was 4 972 580 bp with 36.4% GC content and assembled into 381 scaffolds. No plasmid was detected. A total of 4592 open reading frames (ORFs) were predicted, including 1754 (38%) ORFs annotated as hypothetical proteins and 69 RNAs (one 23S rRNA, one 16S rRNA and four 5S rRNA). Of the 4592 ORFs, 3072 were assigned a putative function (by the Clusters of Orthologous Groups (COGs) database), and 260 genes were identified with an unknown function. Moreover, it contained a complete prophage 57 kb in size and 35.5% GC content (Table 1).
Table 1.
Genome features of Chryseobacterium indologenes MARS15 genome compared to C. indologenes NBRC 14944 and Chryseobacterium sp. stRB126
| Species | Accession No. | Genome size (Mb) | GC% | No. genes | Average nucleotide identity | Source |
|---|---|---|---|---|---|---|
| C. indologenes MARS15 | FCNN01000001-FCNN01000381 | 4.9 | 37.4 | 4592 | — | Pneumonia |
| C. indologenes NBRC 14944 | NZ_BAVL00000000 | 4.75 | 37.2 | 4285 | 99.23 | Pulmonary system |
| C. indologenes J31 | NZ_LAZY00000000 | 5.83 | 36.9 | 5422 | 82.72 | Urine |
| Chryseobacterium sp. stRB126 | NZ_AP014624 | 5.5 | 35.6 | 4961 | 82.57% | Potato |
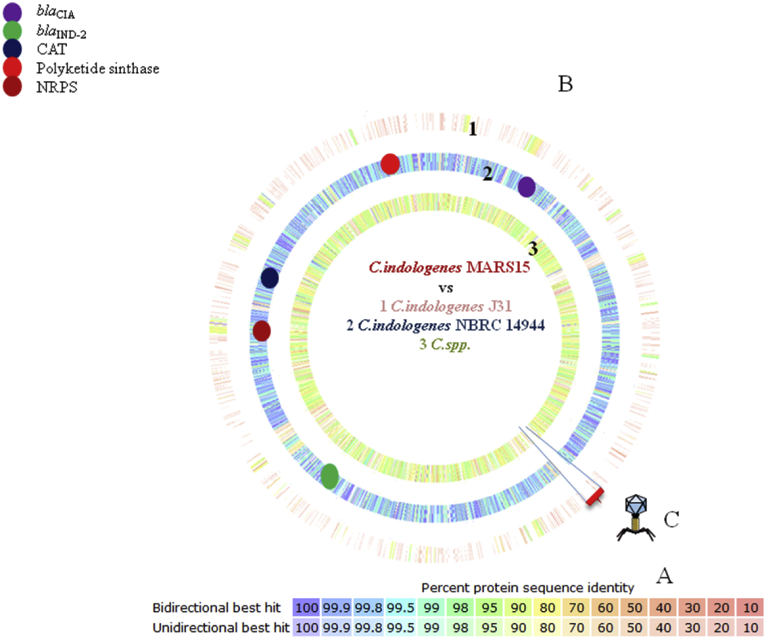
Comparative analysis of genomes of C. indologenes MARS15 reveals more similarity of genome size, GC content, average nucleotide identity (Table 1) and percentage protein sequence identity (Fig. 3) between the clinical strain C. indologenes MARS15 and the reference strain NBRC 14944.
Fig. 3.
Proteomic comparison and in silico DNA-DNA hybridization between MARS15 strain and most closely related species. (A) Colour code referring to percentage of similarity of protein sequence. It refers to average of number of proteins with similarity ≥80% with Chryseobacterium indologenes MARS15 proteome. (B.1) Graphic representation of proteomic comparison between C. indologenes J31 and (B.2) C. indologenes NBRC 14944 and (B.3) Chryseobacterium spp. (C) Representation of prophage absence in other Chryseobacterium analysed.
Resistome
The resistome of this multidrug-resistant C. indologenes MARS15 includes antibiotic resistant genes of the Ambler class A β-lactamase encoding blaCIA and of the Ambler class B β-lactamase blaIND-2, respectively, β-lactamase genes.
The blaCIA gene showed 99% identity with C. indologenes strain NBRC 14944, while the blaIND-2 gene, already reported in Chryseobacterium spp., shared 100% identity with C. indologenes strain NBRC 14944. Moreover, C. indologenes MARS15 possesses four MBL (metallo-β-lactamase) metallohydrolases with unknown activity (Fig. 2(C)). Furthermore, we found a chloramphenicol acetyltransferase encoding CAT, monooxygenase responsible for tetracycline resistance and the multidrug efflux pump AcrB, known to be involved in drug resistance (Fig. 2(C)).
Specific features
Carotenoid biosynthesis
C. indologenes MARS15 has a characteristic yellowish pigmentation on agar plates, caused by the production of carotenoids, as reported for other Chryseobacterium species.
It has been shown that the carotenoid biosynthesis enzyme cluster, including the crtY, crtZ, crtB, crtl and crtX encoding genes, is responsible for the production of the yellow pigment. We found the same arrangement between C. indologenes MARS15 and NBRC 14944, similar in Chryseobacterium strain RB126, but the carotenoid biosynthesis enzyme is absent in C. indologenes J31.
Urease operon
The C. indologenes MARS15 urease gene cluster was composed of seven contiguous genes in a cluster of 4980 bp with 41.4% GC content. The structural genes, ureABC, which encode subunits of the enzyme are flanked immediately upstream by ureD and downstream by the ureEFG encoded urease accessory genes. Overall, hypothetical proteins had no BLAST hits flanking this cluster. The same arrangement is found in C. indologenes strain NBRC 14944 and Chryseobacterium strain RB126. A phylogenetic tree analysis of the concatenated urease gene operons revealed that the urease operon in C. indologenes MARS15 is related to that of Chryseobacterium spp. and Elizabethkingia spp. (data not show).
Phage
In C. indologenes MARS15 we found an intact prophage of 57 kb and 35.5% GC content, whereas incomplete and questionable phages are found, respectively, in C. indologenes strain NBRC 14944 and Chryseobacterium strain RB126; no phages were found in the genome of C. indologenes strain J31 (Fig. 3(C)).
Secondary metabolite biosynthesis
The screening for PKS (Fig. 4) and NRPS revealed one PKS and one cluster of NRPS. The modular PKS, classified as type I, revealed that this cluster encodes erythronolide synthase and β-ketoacyl synthase. The total size of PKS is 11.2 kb, with 43.7 GC%, and a similarity of 37% is found with the Saccharopolyspora erythraea bacterium, already known to be erythromycin producing. Moreover, the analysis of NRPS with unknown activity has an overall average of 12 kb and 41% GC content, encoding thioesterase, AMP binding and condensation starter.
Fig. 4.
Modular organization of polyketide synthase in Chryseobacterium indologenes MARS15, C. indologenes NBRC 14944, C. indologenes STRB126 and Saccharopolispora erythraea NRRL 2338.
Discussion
The genus Chryseobacterium (chry.se.o.bac.teri.um, Gr. adj. chryseos, “golden”) [1], defined in 1994 by Vandamme et al., comprises six species, including C. indologenes (previously Flavobacterium indologenes), which is the most common clinical species in this genus [30]. C. indologenes is an opportunistic pathogen causing nosocomial infections in the presence of an open wound or indwelling device. C. indologenes infection can be lethal in immunocompromised patients [2].
We explored the chromosomally encoded determinants that contribute to the phenotype of resistance of C. indologenes to β-lactams in blaIND-2 and blaCIA genes. These genes conferred, respectively, resistance to amino and carboxypenicillins [31], conferring a narrow spectrum of hydrolysis on penicillins and narrow-spectrum cephalosporins and imipenem.
The 289–amino acid blaCIA shared 99% identity with already reported blaCIA (BAL 40893.1) class A enzymes. The positivity of the Carba NP phenotypic test confirms the presence of carbapenemase production due to the presence of the blaIND-2 gene. Flavin monooxygenase is a vivid example, which demonstrates the enormous adaptability of bacteria: they can freely utilize their protective armor in a large variety of ecological compartments in response to yet another challenge, this time inflicted by humans in the form of antibiotic selective pressure [32]. Some authors suggest that exposure to long-term antibiotics for eradicating bacteria in CF disease select the bacteria, especially multidrug-resistant bacteria, as we have reported elsewhere [33].
C. indologenes shows a characteristic yellowish pigmentation on agar plates, caused by the production of carotenoids, as reported for other Chryseobacterium species [1]. Carotenoid biosynthesis is an ancient process; it is quite plausible that these pigments originally evolved to play a role in membrane stabilization and ultraviolet tolerance [34] and to function as protectors against photodamage, as they are able to quench reactive oxygen species. Therefore, the pigmentation in C. indologenes could play a virulence protective role by allowing a given microbe to evade host immune killing or by provoking inflammatory damage to cells and tissues [35]. The danger of pigmented pathogens might be further heightened in patients with particular immunodeficiencies such as we describe. Carotenoids occur sporadically in nonphotosynthetic bacteria and eukaryotes [36].
Urease is well recognized as an important virulence factor and could be involved in nitrogen assimilation [37], aiding bacterial survival in the acid microenvironment of the inflamed human respiratory tract in patients with chronic obstructive pulmonary disease.
Moreover, the presence of an intact phage in our clinical strain but its absence in the other strain may confer a competitive advantage in chronic lung infection [38].
The ability to produce a secondary metabolite remains silent or inactive under normal laboratory conditions [39]. Therefore, it is difficult to study the mechanisms and signaling pathways behind secondary metabolism activation. Differing from the essential role of a primary metabolite, a secondary metabolite serves instead to increase the fitness of the producing organism or to decrease the fitness of surrounding organisms [40], as is the case of Paenibacillus thiaminolyticus OSY-SE [41], or to adapt to the harsh environment of the inflamed lung.
Conclusion
Here we decipher for the first time by WGS analysis the resistome of C. indologenes, an emerging multidrug-resistant bacterium, from the sputum of a CF patient in France. At the same time, by means of these studies we can map the presence of a biosynthetic gene that could be encoded for novel natural bioactive products. Moreover, thanks to analysis with WGS, we can improve knowledge of multidrug-resistant bacteria and elaborate the mechanism of adaptation of this environmental bacterium to harsh conditions of chronic human infection, such as in CF.
Genome sequence accession number
The genome of C. indologenes MARS15 has been submitted to the European Bioinformatics Institute database under bioproject ID PRJEB12508 with accession number FCNN01000001–FCNN01000381.
Conflict of Interest
None declared.
Acknowledgements
We are grateful to L. Hadjadj for technical assistance and Trad Online for English-language editing. Funded by the French Centre National de la Recherche Scientifique (CNRS) and Infectiopole Sud Foundation.
References
- 1.Vandamme P., Bernardet J.F., Segers P., Kersters K., Holmes B. New perspectives in the classification of the flavobacteria: description of Chryseobacterium gen. nov., Bergeyella gen. nov., and Empedobacter nom. rev. Int J Syst Bacteriol. 1994;44:827–831. [Google Scholar]
- 2.Deng L., Li M.F., Li Y.H., Yang J.L., Zhou X. Chryseobacterium indologenes in four patients with leukemia. Transpl Infect Dis. 2015;17:583–587. doi: 10.1111/tid.12400. [DOI] [PubMed] [Google Scholar]
- 3.Chen X.Y., Zhao R., Chen Z.L., Liu L., Li X.D., Li Y.H. Chryseobacterium polytrichastri sp. nov., isolated from a moss (Polytrichastrum formosum), and emended description of the genus Chryseobacterium. Antonie Van Leeuwenhoek. 2015;107:403–410. doi: 10.1007/s10482-014-0338-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen F.L., Wang G.C., Teng S.O., Ou T.Y., Yu F.L., Lee W.S. Clinical and epidemiological features of Chryseobacterium indologenes infections: analysis of 215 cases. J Microbiol Immunol Infect. 2013;46:425–432. doi: 10.1016/j.jmii.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Nemli S.A., Demirdal T., Ural S. A case of healthcare associated pneumonia caused by Chryseobacterium indologenes in an immunocompetent patient. Case Rep Infect Dis. 2015;2015:483923. doi: 10.1155/2015/483923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirby J.T., Sader H.S., Walsh T.R., Jones R.N. Antimicrobial susceptibility and epidemiology of a worldwide collection of Chryseobacterium spp.: report from the SENTRY Antimicrobial Surveillance Program (1997–2001) J Clin Microbiol. 2004;42:445–448. doi: 10.1128/JCM.42.1.445-448.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omar A., Camara M., Fall S., Ngom-Cisse S., Fall B., Ba-Diallo A. A. Chryseobacterium indologenes in a woman with acute leukemia in Senegal: a case report. J Med Case Rep. 2014;8:138. doi: 10.1186/1752-1947-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu P.C., Chan J.C. Flavobacterium indologenes keratitis. Ophthalmologica. 1997;211:98–100. doi: 10.1159/000310769. [DOI] [PubMed] [Google Scholar]
- 9.Lin J.T., Wang W.S., Yen C.C., Liu J.H., Chiou T.J., Yang M.H. Chryseobacterium indologenes bacteremia in a bone marrow transplant recipient with chronic graft-versus-host disease. Scand J Infect Dis. 2003;35:882–883. doi: 10.1080/00365540310016637. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh P.R., Teng L.J., Yang P.C., Ho S.W., Hsieh W.C., Luh K.T. Increasing incidence of nosocomial Chryseobacterium indologenes infections in Taiwan. Eur J Clin Microbiol Infect Dis. 1997;16:568–574. doi: 10.1007/BF02447918. [DOI] [PubMed] [Google Scholar]
- 11.Christakis G.B., Perlorentzou S.P., Chalkiopoulou I., Athanasiou A., Legakis N.J. Chryseobacterium indologenes non-catheter-related bacteremia in a patient with a solid tumor. J Clin Microbiol. 2005;43:2021–2023. doi: 10.1128/JCM.43.4.2021-2023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kienzle N., Muller M., Pegg S. Chryseobacterium in burn wounds. Burns. 2001;27:179–182. doi: 10.1016/s0305-4179(00)00087-5. [DOI] [PubMed] [Google Scholar]
- 13.Doiz O., Llorente M.T., Mateo A., Seral C., García C., Rubio M.C. Corneal abscess by Flavobacterium indologenes. A case report. Enfermedades Infecc Y Microbiol Clínica. 1999;17:149–150. [PubMed] [Google Scholar]
- 14.Nulens E., Bussels B., Bols A., Gordts B., Van Landuyt H.W. Recurrent bacteremia by Chryseobacterium indologenes in an oncology patient with a totally implanted intravascular device. Clin Microbiol Infect. 2001;7:391–393. doi: 10.1046/j.1198-743x.2001.00273.x. [DOI] [PubMed] [Google Scholar]
- 15.Marcos Sánchez F., Plaza Díaz R., Juárez Ucelay F., Durán Pérez-Navarro A. Pneumonia from Flavobacterium indologenes (group II B) in an immunodepressed patient. An Med Interna. 1993;10:312. [PubMed] [Google Scholar]
- 16.Green B.T., Nolan P.E. Cellulitis and bacteraemia due to Chryseobacterium indologenes. J Infect. 2001;42:219–220. doi: 10.1053/jinf.2001.0822. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira M., Fonseca A.G., Cunha V., Diogo J. Community acquired Chryseobacterium indologenes in an immunocompetent patient. JMM Case Reports. 2014;1 [Google Scholar]
- 18.Beringer P. The clinical use of colistin in patients with cystic fibrosis. Curr Opin Pulm Med. 2001;7:434–440. doi: 10.1097/00063198-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Seng P., Drancourt M., Gouriet F., La Scola B., Fournier P.E., Rolain J.M. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis. 2009;49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 20.Leclercq R., Cantón R., Brown D.F.J., Giske C.G., Heisig P., MacGowan A.P. EUCAST expert rules in antimicrobial susceptibility testing. Clin Microbiol Infect. 2013;19:141–160. doi: 10.1111/j.1469-0691.2011.03703.x. [DOI] [PubMed] [Google Scholar]
- 21.Bakour S., Garcia V., Loucif L., Brunel J.M., Gharout-Sait A., Touati A. Rapid identification of carbapenemase-producing Enterobacteriaceae, Pseudomonas aeruginosa and Acinetobacter baumannii using a modified Carba NP test. New Microbes New Infect. 2015;7:89–93. doi: 10.1016/j.nmni.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aziz R.K., Bartels D., Best A.A., DeJongh M., Disz T., Edwards R.A. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carattoli A., Zankari E., García-Fernández A., Voldby Larsen M., Lund O., Villa L. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S.K., Padmanabhan B.R., Diene S.M., Lopez-Rojas R., Kempf M., Landraud L. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob Agents Chemother. 2014;58:212–220. doi: 10.1128/AAC.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goris J., Konstantinidis K.T., Klappenbach J.A., Coenye T., Vandamme P., Tiedje J.M. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 2007;57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 26.Lagesen K., Hallin P., Rødland E.A., Staerfeldt H.H., Rognes T., Ussery D.W. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y., Liang Y., Lynch K.H., Dennis J.J., Wishart D.S. PHAST: a fast phage search tool. Nucleic Acids Res. 2011;39:W347–W352. doi: 10.1093/nar/gkr485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drissi F., Buffet S., Raoult D., Merhej V. Common occurrence of antibacterial agents in human intestinal microbiota. Front Microbiol. 2015;6:441. doi: 10.3389/fmicb.2015.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber T., Blin K., Duddela S., Krug D., Kim H.U., Bruccoleri R. AntiSMASH 3.0—a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015;43:W237–W243. doi: 10.1093/nar/gkv437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellais S., Poirel L., Leotard S., Naas T., Nordmann P. Genetic diversity of carbapenem-hydrolyzing metallo-beta-lactamases from Chryseobacterium (Flavobacterium) indologenes. Antimicrob Agents Chemother. 2000;44:3028–3034. doi: 10.1128/aac.44.11.3028-3034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto T., Nagata M., Ishimine N., Kawasaki K., Yamauchi K., Hidaka E. Characterization of CIA-1, an Ambler class A extended-spectrum β-lactamase from Chryseobacterium indologenes. Antimicrob Agents Chemother. 2012;56:588–590. doi: 10.1128/AAC.05165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aminov R.I. Evolution in action: dissemination of tet(X) into pathogenic microbiota. Front Microbiol. 2013;4:192. doi: 10.3389/fmicb.2013.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bittar F., Leydier A., Bosdure E., Toro A., Reynaud-Gaubert M., Boniface S. Inquilinus limosus and cystic fibrosis. Emerg Infect Dis. 2008;14:993–995. doi: 10.3201/eid1406.071355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klassen J.L. Phylogenetic and evolutionary patterns in microbial carotenoid biosynthesis are revealed by comparative genomics. PLoS One. 2010;5:e11257. doi: 10.1371/journal.pone.0011257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu G.Y., Nizet V. Color me bad: microbial pigments as virulence factors. Trends Microbiol. 2009;17:406–413. doi: 10.1016/j.tim.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson E.A., Schroeder W.A. Microbial carotenoids. Adv Biochem Eng Biotechnol. 1996;53:119–178. doi: 10.1007/BFb0102327. [DOI] [PubMed] [Google Scholar]
- 37.Mobley H.L., Hausinger R.P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989;53:85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.James C.E., Davies E.V., Fothergill J.L., Walshaw M.J., Beale C.M., Brockhurst M.A. Lytic activity by temperate phages of Pseudomonas aeruginosa in long-term cystic fibrosis chronic lung infections. ISME J. 2015;9:1391–1398. doi: 10.1038/ismej.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nützmann H.W., Reyes-Dominguez Y., Scherlach K., Schroeckh V., Horn F., Gacek A. Bacteria-induced natural product formation in the fungus Aspergillus nidulans requires Saga/Ada-mediated histone acetylation. Proc Natl Acad Sci U S A. 2011;108:14282–14287. doi: 10.1073/pnas.1103523108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brakhage A.A. Regulation of fungal secondary metabolism. Nat Rev Microbiol. 2013;11:21–32. doi: 10.1038/nrmicro2916. [DOI] [PubMed] [Google Scholar]
- 41.Huang E., Guo Y., Yousef A.E. Biosynthesis of the new broad-spectrum lipopeptide antibiotic paenibacterin in Paenibacillus thiaminolyticus OSY-SE. Res Microbiol. 2014;165:243–251. doi: 10.1016/j.resmic.2014.02.002. [DOI] [PubMed] [Google Scholar]