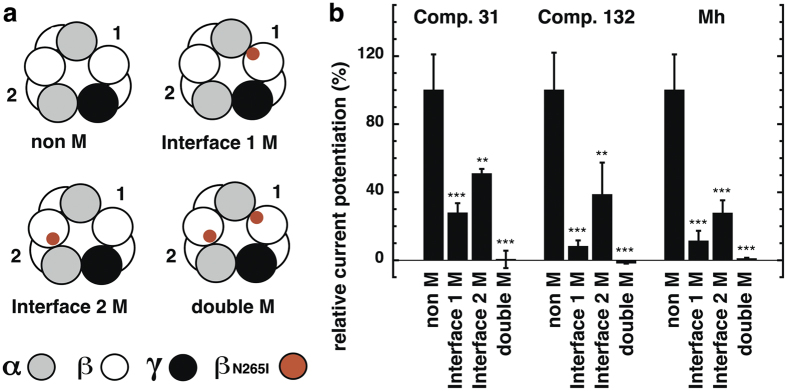
Figure 5. Individual roles of the two β+/α− interfaces in channel modulation by compounds 31, 132 and 4-O-methylhonokiol.
(a) Scheme showing the four concatenated wild-type and mutant receptors. 1 and 2 refer to the two different β+/α− subunit interfaces, interface 1 and interface 2. The location of the β2N265I mutations is indicated in red color. Concatenated receptors were prepared containing no mutation (α1-β2-α1/γ2-β2, non M), a mutation at interface 1 (α1-β2-α1/γ2-β2M, interface 1 M), a mutation at interface 2 (α1-β2M-α1/γ2-β2, interface 2 M), or mutations in both sites (α1-β2M-α1/γ2-β2M, double M). Interface 2 harbors a binding site for GABA with higher apparent affinity for channel gating than the one positioned at the interface 154. (b) Potentiation by compound 31 (3 μM), compound 132 (3 μM), and 4-O-methylhonokiol (1 μM), using an EC0.5–1.5 concentration of GABA for each concatenated receptor subtype. Bars indicate mean ± SD, n = 3.