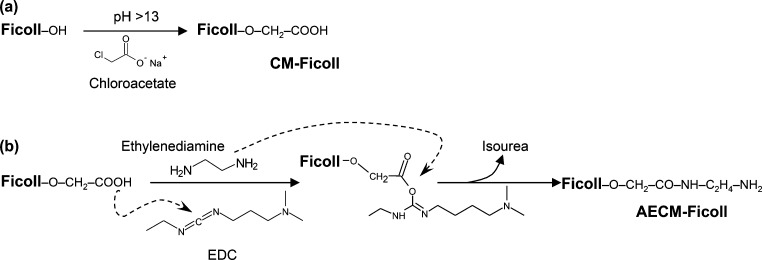
Figure 2.
Derivatization of Ficoll with aminoethyl groups. (a) Ficoll is reacted with chloroacetate sodium salt at basic pH at 40 °C for 2.5 h, followed by rapid cooling and pH neutralization with chloroacetic acid in order to stop the derivatization reaction, resulting in formation of CM-Ficoll. (b) Excess ethylenediamine is added to CM-Ficoll at pH 4.5, followed by slow addition of excess of EDC, leading to formation of a short-lived but highly reactive o-acylisourea intermediate. This reactive ester reacts with ethylenediamine to form a stable amide bond leading to formation of AECM-Ficoll, with release of isourea as byproduct.