Abstract
Objectives
To prospectively determine clinical and biochemical characteristics associated with the development of peripheral neuropathy, loss of protective sensation, and foot ulceration in persons with type 2 diabetes mellitus (DM) over 7 years.
Research design and methods
Graded monofilament (MF) testing, vibration perception threshold, and neuropathy symptom questionnaires were undertaken in 206 participants with type 2 DM without peripheral vascular disease or history of foot ulceration and 71 healthy participants without DM at baseline and after 7 years. 6 monthly glycosylated hemoglobin (HbA1c) levels and annual serum lipid profiles were measured during follow-up of those with DM. Incident foot ulceration was recorded at follow-up.
Results
Taller stature and higher quartiles of serum triglyceride and HbA1c levels were associated with neuropathy at follow-up (p=0.008). Remission of baseline neuropathy was observed in 7 participants at follow-up. 9 participants with type 2 DM developed foot ulcers by the end of the study, only 1 at low risk. Mean HbA1c levels were higher in those who developed foot ulceration (p<0.0001). 1 participant with neuropathy throughout developed a Charcot foot. Failure to perceive 2 or more 2, 4 and 6 g MF stimuli at baseline predicted loss of protective sensation at follow-up.
Conclusions
Tall stature and worse metabolic control were associated with progression to neuropathy. Mean HbA1c levels were higher in those who developed foot ulcers. Graded MF testing may enrich recruitment to clinical trials and assignation of high risk for foot ulceration.
Keywords: Peripheral Neuropathy, Type 2 Diabetes, Dyslipidemia, Glycemic Control
Key messages.
Quantifiable interaction of stature and metabolic control in risk for neuropathy.
Poor long-term glycemic control associated with foot ulceration.
Impaired monofilament (MF) perception at all weights in diabetic compared with control group.
Predictive value of graded MFs in development of protective sensory loss.
Introduction
Clinical evaluation of pedal sensation in patients with diabetes mellitus (DM) using vibration perception threshold (VPT) and sensing of monofilament (MF) stimuli can identify those at increased risk of foot ulceration and consequent lower extremity amputation.1–5 These tests are often combined with presence of neuropathic symptoms to diagnose peripheral neuropathy.6 They are all subjective, easily and cheaply applied in routine and research clinics. However, they are not always well performed in routine screening. In clinical practice, failure to perceive one or more 10 g MF stimuli is used to assign high risk of foot ulceration and referral for community podiatry follow-up (National Institute for Health and Care Excellence (NICE) clinical guideline 10 guidance.nice.org.uk/cg10). In view of the proven predictive value of these assessments, it is of concern that general practice and ward testing of feet in patients with DM may be performed imprecisely or not at all. The use of graded MFs may also allow identification of a high-risk group with early loss of sensation not amounting to neuropathy.7 8 Minor degrees of pedal sensory loss, if progression to loss of protective sensation were proven, would allow such patients to be referred to community podiatry follow-up rather than annual review.1 5 Identification of a high-risk group at an earlier stage of the complication would also enrich recruitment to clinical trials of neuropathy. Progress in ulcer prevention has been slow, and further studies of well-characterized participants are much needed.9 The present study was designed to assess the interaction between (1) known risk factors for development of neuropathy and foot ulceration and (2) predictive value of graded MF perception in prediction of loss of protective sensation.10–13
Methods
Ethical approval: South West Regional Ethics Committee permission was granted to perform symptom questionnaire (SQ) and neurological testing of participants with and without type 2 DM both at baseline and at follow-up.
Recruitment: Study recruitment and baseline assessment took place between 1998 and 2001 at Torbay Hospital diabetic outpatient department. Participants without DM were recruited from hospital staff and their relatives at South Devon Healthcare National Health Service (NHS) Foundation Trust. The study started at the time of presentation of the UK Prospective Diabetes Study (UKPDS) results for which Torbay had been a center.14 In the light of those results, patients were referred to secondary care DM for diet therapy and consideration of statins. The study population was recruited from this group. Those who admitted on questioning excess alcohol consumption, history of chemotherapy, or vitamin deficiency were excluded (five persons). Inclusion criteria were age 40–75 years; confirmed type 2 DM without ketosis; capacity to participate in testing routine; willingness to return for follow-up testing (24 persons with DM were excluded—19 failed to attend the screening visit, 2 were heavy alcohol consumers, 1 had cervical myelopathy, 2 had ongoing cancer treatment).
Neurological testing: Results from a previously published comparator group without DM were used to establish the sensory testing protocol.7 MF testing was performed at pulp of hallux, and first, second, third and fifth metatarsal heads of each foot in all participants (total of 10 sites) after removal of callus. Participants were reclining comfortably in a quiet room at 15–18°C with eyes closed. Testing began with 2 g MF and continued with increasing weights up to 15 g. The MF was applied to the test site and pressed until buckling. MFs were calibrated at baseline, 3 and 6 years (Bailey instruments, Manchester, UK). VPT was tested applying light pressure to the pulp of the hallux, taking an average of three voltages at which vibration was perceived using one neurothesiometer which was calibrated annually. The entire non-diabetic comparator group had VPT<15 V in both feet providing a cut-off for abnormality. A standard set of questions was then asked to elicit symptoms of tingling, shooting pains and night pains in the feet and legs, validated in a previous study7 consistent with other published data.11 15–19
Diabetic peripheral sensory neuropathy was defined as at least two of:
Failure to perceive the 10 g MF at 1 or more of 10 test sites;
VPT>15 V in both feet;
Symptoms typical of diabetic neuropathy such as night pain, tingling, or shooting pains in both feet.
Two healthcare professionals, a research nurse and research registrar were trained by the podiatrist to perform sensory testing, exactly as had been performed at baseline. All tests were performed by these three investigators. Interobserver agreement was excellent classifying those with normal sensation as normal in all cases (n=10), and all neuropaths correctly (n=10).
Other clinical evaluation: Height and ankle-brachial pressure index were measured at baseline. Weight, mean of three sitting blood pressure readings, and foot pulses assessed by palpation and hand-held Doppler were measured at each visit.
Blood tests: Glycosylated hemoglobin (HbA1c) was measured at baseline then at least twice yearly by a Diabetes Control and Complications Trial (DCCT)-validated method. Serum creatinine, glucose, and lipid profiles were measured annually by standard laboratory methods. Over 90% of the participants with type 2 DM were started on statin therapy during the first year of the study, serum cholesterol 5.5±1.1 at baseline versus 4.0±0.9 mmol/L at final follow-up.
Statistical methods
Sample size: The study was designed as a descriptive study of foot sensory testing, neuropathy incidence, and ulcer development in type 2 diabetic participants without peripheral vascular disease. A similar size cohort was studied in the Seattle study of neuropathy incidence.2 In Torbay, the incidence of new foot ulceration was 1.64% in 1999 which amounts to a probability of 16 ulcers in 200 patients over 5 years.20 Double inputting of data was performed by a clerical officer unaware of the potential correlations between variables. Comparison between groups of normally distributed variables was made with unpaired t test. Serum triglyceride levels were log transformed before analysis. The χ2 testing was used for proportion with and without significant neurological test results. The interaction and residual significance of multiple potential risk factors for progression to neuropathy was tested in a general linear model (GLM). Variables included at baseline were gender, age, duration of DM, height, weight, body mass index, serum cholesterol, natural log-transformed serum triglycerides, HbA1c. All were also combined in all possible two-way interactions. The dependent variable was the binomial data for final presence or absence of neuropathy. A χ2 based drop function to remove insignificant interactions and redistribute variance was then used in a general linear model with binomial link function as in a previous study.21 22 Mean updated serum triglyceride and HbA1c levels were expressed as above or below the mean with χ2 analysis to assess differences in progression to neuropathy.14
Spline analysis was used to compare long-term diabetic glycemic control as mean six monthly updated HbA1c levels in participants who developed foot ulceration during follow-up and those who did not.
Results
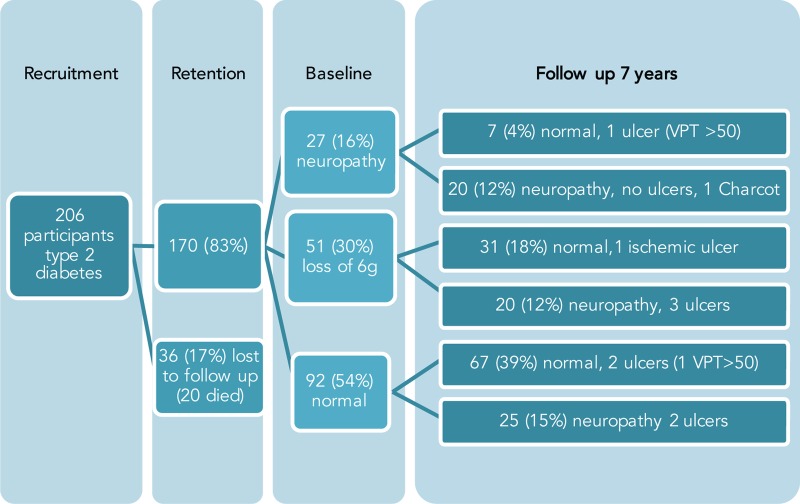
Recruitment number, retention and outcome are shown in figure 1.
Figure 1.
Changes in neurological testing, neuropathy status, and foot ulceration in 170 participants with type 2 DM over 7 years of follow-up. DM, diabetes mellitus; VPT, vibration perception threshold.
Baseline results: The diabetic and non-diabetic groups were well matched for gender and age, but those with type 2 DM had higher weight and HbA1c, and lower serum high-density lipoprotein cholesterol. Analysis of variance and χ2 tests showed that results of MF testing, VPT, and SQ were distinct in the two groups as shown in table 1.
Table 1.
Anthropometric, metabolic, and sensory testing data in type 2 diabetic and comparison non-diabetic participants at baseline
| Baseline results | Type 2 diabetes | No diabetes | Significance |
|---|---|---|---|
| Number | 206 | 71 | |
| Age, mean years (SD) | 61.0 (8.4) | 60.1 (10.7) | NS |
| Women (%) | 39.4 | 40.7 | NS |
| Ethnic origin | 99.4% white British | 97% white British | NS |
| Duration of diabetes months (SD) | 62.3 (range 1–190) | – | |
| Height, cm (SD) | 170.0 (9.9) | 172.5 (9.5) | NS |
| Weight, kg (SD) | 90.9 (17.5) | 76.7 (14.7) | p<0.001 |
| BMI, kg/m2 (SD) | 31.3 (5.6) | 25.5 (3.4) | p<0.001* |
| Blood pressure, mm Hg, systolic (SD) | 138.0 (19.0) | 128.3 (16.9) | p<0.001* |
| Blood pressure, mm Hg, diastolic (SD) | 79.3 (9.9) | 79.5 (11.8) | NS |
| Cholesterol, mmol/L(SD) | 5.5 (1.1) | 5.4 (0.9) | NS |
| HDL cholesterol, mmol/L (SD) | 1.26 (0.26) | 1.8 (1.1) | 0.007* |
| Triglycerides, mmol/L, median (IQR) | 2.0 (1.4–2.9) | 1.4 (0.4–2.1) | 0.091† |
| ABPI (SD) | 1.1 (0.11) | Not tested | |
| Mean right VPT (SD) | 16.7 (10.2) | 9.1 (4.7) | p<0.001* |
| Mean left VPT (SD) | 16.1 (10.6) | 9.6 (5.1) | p<0.001* |
| Number failed >1 10 g MF | 39 of 206 | 0 of 71 | p<0.001‡ |
| Number failed >2 6 g MF | 51 of 206 | 3 of 71 | p<0.001‡ |
| Number neuropathic symptoms | 11 of 206 | 0 of 71 | p<0.001‡ |
*Two-tailed t test unequal variance.
†Log-transformed data.
‡χ2 test.
ABPI, ankle-brachial pressure index; BMI, body mass index; HDL, high-density lipoprotein; MF, monofilament; NS, not significant; VPT, vibration perception threshold.
The differences in graded MF sensation between diabetic and non-diabetic groups at baseline and follow-up are shown in table 2. Loss of perception of two or more 2, 4, 6, and 8 g MF was significantly more common in the diabetic participants at baseline and follow-up compared with the non-diabetic group (loss of perception of one 2 g MF did not discriminate between diabetic and non-diabetic subjects). Patients with loss of 10 g perception at baseline were excluded.
Table 2.
Baseline 2, 4, 6, and 8 g MF insensitivity (with normal 10 g at baseline) and association with 10 g loss at follow-up
| MF grade | 2 | 4 | 6 | 8 | MF grade | 2 | 4 | 6 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| DM baseline loss >1 MF (10 g normal) | 113 | 58 | 40 | 29 | DM baseline normal/one loss (10 g normal) | 30 | 81 | 99 | 122 |
| FU 10 g loss | 17 | 18 | 14 | 22 | FU 10 g loss | 3 | 11 | 15 | 21 |
| Percent prediction of 10 g loss | 15 | 31 | 35 | 76 | Percent prediction of 10 g loss | 10 | 13.5 | 15 | 17 |
| χ2≥2 losses vs 1 or 0 | 0.4 | 0.013 | 0.009 | 0.0001 |
Patients with loss of 10 g perception at baseline excluded.
DM, diabetes mellitus; FU, follow-up; MF, monofilament.
Online supplementary table S1 baseline graded MF results in diabetic and non-diabetic comparator groups. Patients with loss of 10 g perception at baseline excluded.
bmjdrc-2015-000163supp_table1.pdf (15KB, pdf)
Follow-up data: None of the group without DM developed frank neuropathy or isolated loss of 10 g MF sensation. Two described symptoms which conformed to the SQ definition of neuropathic pain and two others developed an increase in VPT to >15 V bilaterally, one of whom had developed type 2 DM, fasting blood glucose 9 mmol/L.
Table 2 showing the predictive value of baseline loss of perception of two or more stimuli at 2, 4, 6, and 8 g weights of MF (patients with baseline loss of 10 g perception excluded).
One participant with type 2 DM and neuropathy throughout the study developed a Charcot foot after minimal trauma as did one patient with baseline neuropathy lost to study follow-up. Nine of those with type 2 DM developed foot ulceration—two with bilateral VPT>50 V only, one developed ischemia without neuropathy, five developed neuropathy during follow-up, and one had no additional risk factors (figure 1). All of the patients with ulcer survived until the end of the study and none proceeded to amputation. Their characteristics and timing of ulcer development are shown in online supplementary table S2. Two were single divorced males living alone, and the other seven living with partners and comfortably off.
bmjdrc-2015-000163supp_table2.pdf (27.1KB, pdf)
General linear modeling of age, duration of DM, anthropometric and metabolic variables identified only height (p<0.0018), and mean updated serum triglycerides (p<0.029) as significant in development of neuropathy at follow-up. Metabolic results expressed as greater or less than updated mean values of HbA1c and serum triglycerides are shown in figure 2. Diabetic participants below mean updated HbA1c and serum triglycerides were found to have less neuropathy at follow-up as shown in figure 2 (p=0.008).
Figure 2.
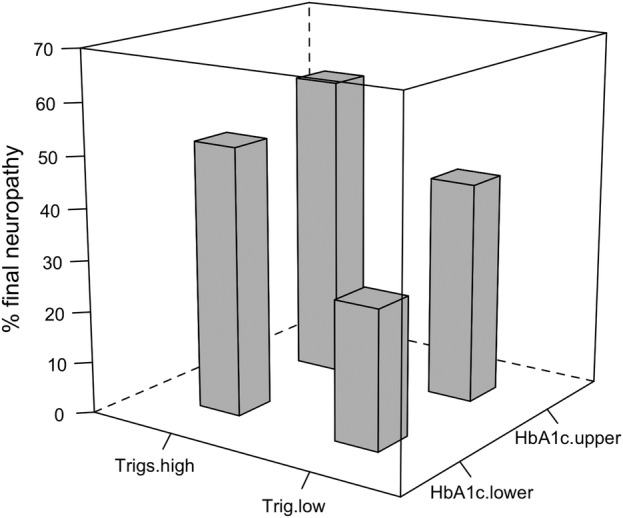
Interaction of updated mean serial HbA1c and serum triglyceride levels with sensory peripheral neuropathy over 7 years in 151 type 2 diabetic participants. Z and X axis division of groups is taken from mean triglyceride 2.1 mmol/L and mean HbA1c 62.8 mmol/mol (7.9%) respectively. HbA1c, glycosylated hemoglobin.
The modeled probability of developing neuropathy at different heights with increasing triglyceride levels is shown in online supplementary figure S1. Model summary: residual deviance/Degrees of Freedom (DOF)=1.197, F statistic=0.8586 on 2 and 106 DOF, adjusted R2 −0.0026.
The nine diabetic participants who developed foot ulceration during the study had similar age, gender, height, and triglyceride levels to those who did not. However, their serial HbA1c levels were higher at each time point except one, and a spline analysis confirmed significantly higher glycemia in the nine participants who developed foot ulceration compared with the rest of the diabetic cohort (figure 3, overall p<0.0001, R2=0.0125, t=4.35).
Figure 3.
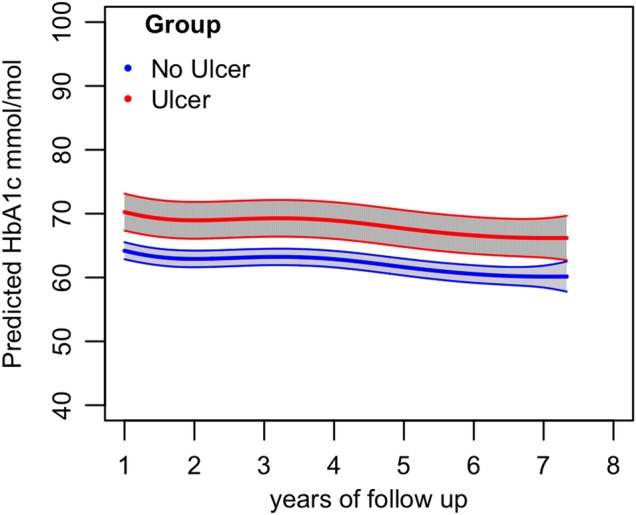
Difference in HbA1c between type 2 diabetic participants with ulcers versus no ulcers on GLM analysis with spline fitted to time (p<0.0001, R2=0.0125, t=4.35). HbA1c, glycosylated hemoglobin.
Online supplementary tables S2–S4 show the details of the nine patients with ulcer, five deceased participants with neuropathy at baseline, and the seven whose neuropathy remitted, respectively. Their baseline characteristics did not differ significantly from the main cohort.
bmjdrc-2015-000163supp_table3.pdf (26.5KB, pdf)
bmjdrc-2015-000163supp_table4.pdf (32.2KB, pdf)
Discussion
Graded MF, VPT, and SQ results in the diabetic cohort were distinct from the comparator group without DM. The normality and stability of pedal sensory testing results and rarity of typical neuropathic symptoms in the group without DM was maintained at 7 years. In contrast, only 30 diabetic participants at baseline and 21 of these at follow-up preserved 2 g MF perception. It is also notable that 7 of 27 participants with neuropathy at baseline remitted after 7 years—a finding recently reported in other studies of nerve conduction and vibration perception.12 13 The follow-up results quantify the interactions between height and serial metabolic control in development of neuropathy. Our study extends the observations of previous studies in this group of purely type 2 diabetic participants without peripheral vascular disease, or previous ulceration and including serial metabolic testing, recording of foot ulceration and a comparator non-diabetic group.2 5 19 Of the nine participants who developed foot ulceration, only one lacked any risk factors other than the presence of DM. However, two had only impaired VPT, and two only ischemia. This emphasizes the distinction between sensory loss in assigning risk of foot ulceration, and formal diagnosis of neuropathy which has conventionally relied on a double check of two characteristics of disturbed sensation. Loss of perception of two or more 2–8 g MF tests was significantly greater in the diabetic participants which implies widespread sensory dysfunction in a large proportion of this group of type 2 diabetic participants (138 of 168 at baseline). These changes also predicted impairment of protective sensation 7 years later. One study of children with type 1 DM has confirmed a strong correlation between research standard graded MF perception and nerve conduction studies in identification of early neuropathy but long-term follow-up was not undertaken.23 Association of dyslipidemia, vascular disease, and glycemia in development of neuropathy in type 1 DM has been demonstrated.24 In order to replicate these findings in DM annual review, research grade MF and a rigorous standard operating procedure would be necessary. The strength of our study derives from the well-defined characteristics of the diabetic cohort; graded MF assessment; and follow-up of sensory loss, neuropathy and ulcer incidence. Clinical notes were searched for record of ulceration in those lost to trial follow-up. One developed a Charcot foot but no foot ulcers were recorded. Other potential confounders include socioeconomic factors, nutrition, and any effects of DM-related therapy. The first of these has been shown to affect amputation rate and ulcer prevalence rather than neuropathy incidence. We did not measure linolenic acid intake which has been shown to be associated with diabetic peripheral neuropathy prevalence. Statin therapy has been shown to improve DM complication rate including amputation.25–28 A weakness is that the cohort consisted exclusively of white British participants. However, this ethnic group is at the highest risk of diabetic foot ulceration and lower extremity amputation in the UK.29 The number of incident ulcers was only nine, implying that a larger cohort should be recruited for more comprehensive analysis of ulcer development. Validation of neuropathy status by nerve conduction studies was not available to us or practicable in such a large group. However, prediction of foot ulceration has been shown to be closely linked to loss of MF perception and or VPT. Both of these measurements and SQs are readily and cheaply accessible in a wide range of healthcare settings but demand adequate training to be delivered with precision.
Associations between serial HbA1c levels and outcomes related to diabetic peripheral neuropathy have been reported in participants with type 2 DM in several studies,12 13 30 31 only one of which found a reduction in progression with improved glycemia. Neuropathy in patients with type 1 DM in the DCCT was significantly reduced in the intensive glycemic control group.32 Higher serial triglyceride levels in those who progressed to neuropathy modified the strong predictive value of height in the linear model in our study. Ninety percent of our participants were treated with statins which resulted in an improvement in serum cholesterol levels sustained over the 7-year follow-up period. Although there was no metabolic threshold below which neuropathy did not occur, those below mean updated HbA1c and serum triglyceride levels had a lower percentage neuropathy at follow-up. This is consistent with the hypothesis that the severity of the metabolic disturbance in DM influences susceptibility to neuropathy. There is a possibility that intense reduction in glycemia from diagnosis could improve peripheral nerve function.32 Similarly, an intervention to normalize serum triglycerides might have beneficial effects on development of neuropathy.33 Increase in serum triglyceride levels were significantly associated with 10-year lower extremity amputation rates in a large cohort of patients with predominantly type 2 DM in the DISTANCE study.33 However, in our study participants who developed foot ulcers did not have significantly higher lipid levels than those who did not. The ulcer group did have higher HbA1c levels which might have increased susceptibility to ulceration. It is also likely that environmental or genetic factors unrelated to glycemia or lipid trafficking contribute to susceptibility to neuropathy. A clear example of this in our study was height. The increased risk of diabetic peripheral neuropathy in relation to stature is striking and was first shown in 1988.10 Body stature should be taken into account as suggested in modeling of the risk factors, such as serum triglyceride levels in the present study (see online supplementary figure S1). In addition, at least one example of undefined determinants of susceptibility to peripheral diabetic neuropathy has been described. This concerns the striking absence of peripheral neuropathy in Alström syndrome participants despite severe hyperglycemia and hypertriglyceridemia from adolescence compared with weight and height and age-matched persons with onset of type 2 DM in adolescence.34
bmjdrc-2015-000163supp_figure.pdf (75.2KB, pdf)
In conclusion, this study has confirmed that current subjective clinical sensory testing by healthcare professionals, trained by the podiatrist to perform sensory testing, can reliably evaluate progression and remission of clinically determined peripheral sensory neuropathy in those with type 2 DM. In particular, the data may be used to perform power calculations preparatory to interventional studies in neuropathy and foot ulcer prevention. Loss of lesser weight MF perception, not currently accepted as diagnostic of neuropathy or high risk does predict subsequent loss of 10 g MF perception. If confirmed, this finding may be incorporated in to clinical trials of early neuropathy and broaden the diagnostic criteria for high ulceration risk. These results endorse the need for foot sensory testing to be performed well, particularly in general practice annual diabetic review, where training of all staff involved and a standard operating procedure are strongly recommended (Putting Feet First-diabetes.org.uk). HbA1c levels and mean serum triglyceride levels were potentially modifiable risk factors and height a crucial inherent predictor of neuropathy. There are also likely to be as yet undiscovered genetic or environmental influences which determine susceptibility to diabetic peripheral neuropathy. Mean HbA1c levels were higher in those who developed foot ulceration.
Acknowledgments
The authors would like to thank Lynne Bower, Richard Stanton, and Jonathan Drew for biochemical measurements, results retrieval, and data in-putting, respectively. They are also sincerely grateful to the patients for their enthusiastic participation. The authors would also like to thank Melvin Cowie for graphic design of the figures.
Footnotes
Contributors: RBP designed the study, researched the data and wrote the manuscript. TD researched the study and contributed to the manuscript. AMG researched the study and contributed to the manuscript. MW advised on biochemistry, coordinated, stored and analyzed the samples. PH advised on statistical analysis and writing the manuscript. CFP advised on and performed statistical analysis and contributed to the manuscript. MPT carried out the pilot study, designed the present study, trained researchers and contributed to the manuscript.
Funding: This study was funded by the Torbay Special Medical Projects Fund.
Competing interests: None declared.
Ethics approval: South West Regional Ethics Committee, UK.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Hard copies of patient clinical and study notes have been retained for 15 years. The source data in excel files have been anonymized and stored in secure servers under the aegis of South Devon Healthcare NHS Foundation Trust.
References
- 1.Kumar S, Fernando DJ, Veves A et al. . Semmes-Weinstein monofilaments: a simple, effective and inexpensive screening device for identifying diabetic patients at risk of foot ulceration. Diabetes Res Clin Pract 1991;13:63–7. 10.1016/0168-8227(91)90034-B [DOI] [PubMed] [Google Scholar]
- 2.Adler AI, Boyko EJ, Ahroni JH et al. . Risk factors for diabetic peripheral sensory neuropathy: results of the Seattle prospective diabetic foot study. Diabetes Care 1997;20:1162–7. 10.2337/diacare.20.7.1162 [DOI] [PubMed] [Google Scholar]
- 3.Pham H, Armstrong DG, Harvey C et al. . Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care 2000;23:606–11. 10.2337/diacare.23.5.606 [DOI] [PubMed] [Google Scholar]
- 4.Kamei N, Yamane K, Nakanishi S et al. . Effectiveness of Semmes-Weinstein monofilament examination for diabetic peripheral neuropathy screening. J Diabetes Complications 2005;19:47–53. 10.1016/j.jdiacomp.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 5.Boyko EJ, Ahroni JH, Cohen V et al. . Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care 2006;29:1202–7. 10.2337/dc05-2031 [DOI] [PubMed] [Google Scholar]
- 6.Meijer JWG, Smit AJ, Sonderen EV et al. . Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the Diabetic Neuropathy Symptom score. Diabet Med 2002;19:962–5. 10.1046/j.1464-5491.2002.00819.x [DOI] [PubMed] [Google Scholar]
- 7.Thomson MP, Potter J, Finch PM et al. . Threshold for detection of diabetic peripheral sensory neuropathy using a range of research grade monofilaments in persons with type 2 diabetes mellitus. J Foot Ankle Res 2008;1:9 10.1186/1757-1146-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perkins BA, Orszag A, Ngo M et al. . Prediction of incident diabetic neuropathy using the monofilament examination: a 4-year prospective study. Diabetes Care 2010;33:1549–54. 10.2337/dc09-1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer S. Pressure relieving interventions for preventing and treating diabetic foot ulcers. Cochrane Database Syst Rev 2000;(3):CD002302 10.1002/14651858.CD002302 [DOI] [PubMed] [Google Scholar]
- 10.Sosenko JM, Boulton AJ, Gadia MT et al. . The association between symptomatic sensory neuropathy and body stature in diabetic patients. Diabetes Res Clin Pract 1988;4:95–8. 10.1016/S0168-8227(88)80003-2 [DOI] [PubMed] [Google Scholar]
- 11.Franklin GM, Kahn LB, Baxter J et al. . Sensory neuropathy in non-insulin-dependent diabetes mellitus. The San Luis Valley Diabetes Study. Am J Epidemiol 1990;131:633–43. [DOI] [PubMed] [Google Scholar]
- 12.Fujita Y, Fukushima M, Suzuki H et al. . Short-term intensive glycemic control improves vibratory sensation in type 2 diabetes. Diabetes Res Clin Pract 2008;80:e16–19. 10.1016/j.diabres.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 13.Perkins BA, Dholasania A, Buchanan RA et al. . Short-term metabolic change is associated with improvement in measures of diabetic neuropathy: a 1-year placebo cohort analysis. Diabet Med 2010;27:1271–9. 10.1111/j.1464-5491.2010.03110.x [DOI] [PubMed] [Google Scholar]
- 14.Stratton IM, Adler AI, Neil HAW et al. . Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–12. 10.1136/bmj.321.7258.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkins BA, Olaleye D, Zinman B et al. . Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care 2001;24:250–6. 10.2337/diacare.24.2.250 [DOI] [PubMed] [Google Scholar]
- 16.Vinik EJ, Hayes RP, Oglesby A et al. . The development and validation of the Norfolk QOL-DN, a new measure of patients’ perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther 2005;7:497–508. 10.1089/dia.2005.7.497 [DOI] [PubMed] [Google Scholar]
- 17.Abbott CA, Malik RA, van Ross ERE et al. . Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011;34:2220–4. 10.2337/dc11-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spallone V, Morganti R, D'Amato C et al. . Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet Med 2012;29:578–85. 10.1111/j.1464-5491.2011.03500.x [DOI] [PubMed] [Google Scholar]
- 19.Bril V, Tomioka S, Buchanan RA et al. . Reliability and validity of the modified Toronto Clinical Neuropathy Score in diabetic sensorimotor polyneuropathy. Diabet Med 2009;26:240–6. 10.1111/j.1464-5491.2009.02667.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon S. A review of: ‘Statistical Rules of Thumb, Second Edition, by G. van Belle’: Hoboken: Wiley, 2008, ISBN 978-0-470-14448-0, xxi + 272 pp., $59.95. J Biopharm Stat 2009;19:752–4. 10.1080/10543400902964217 [DOI] [Google Scholar]
- 21.Paisey RB, Smith J, Carey C et al. . Duration of diabetes predicts aortic pulse wave velocity and vascular events in Alström syndrome. J Clin Endocrinol Metab 2015;100:E1116–24. 10.1210/jc.2015-1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. An Application of Gamma Generalized Linear Model for Estimation of Survival Function of Diabetic Nephropathy Patients Gurprit Grover, Alka Sabharwal* and Juhi Mittal—Norton Safe Search. (cited 12 January 2016). http://nortonsafe.search.ask.com/web?q=An+Application+of+Gamma+Generalized+Linear+Model+for+Estimation+of+Survival+Function+of+Diabetic+Nephropathy+Patients+Gurprit+Grover%2C+Alka+Sabharwal*+and+Juhi+Mittal&geo=GB&prt=NSBU&locale=en_GB&o=APN10505&chn=retail&ver=22&tpr=2&ts=1452592694785.
- 23.Blankenburg M, Kraemer N, Hirschfeld G et al. . Childhood diabetic neuropathy: functional impairment and non-invasive screening assessment. Diabet Med 2012;29:1425–32. 10.1111/j.1464-5491.2012.03685.x [DOI] [PubMed] [Google Scholar]
- 24.Tesfaye S, Chaturvedi N, Eaton SEM et al. . Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–50. 10.1056/NEJMoa032782 [DOI] [PubMed] [Google Scholar]
- 25.Reiber GE, Pecoraro RE, Koepsell TD. Risk factors for amputation in patients with diabetes mellitus. A case-control study. Ann Intern Med 1992;117:97–105. 10.7326/0003-4819-117-2-97 [DOI] [PubMed] [Google Scholar]
- 26.Haji Zaine N, Burns J, Vicaretti M et al. . Characteristics of diabetic foot ulcers in Western Sydney, Australia. J Foot Ankle Res 2014;7:39 10.1186/s13047-014-0039-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao M, McDowell MA, Saydah SH et al. . Relationship of polyunsaturated fatty acid intake to peripheral neuropathy among adults with diabetes in the National Health and Nutrition Examination Survey (NHANES) 1999 2004. Diabetes Care 2008;31:93–5. 10.2337/dc07-0931 [DOI] [PubMed] [Google Scholar]
- 28.Davis TME, Yeap BB, Davis WA et al. . Lipid-lowering therapy and peripheral sensory neuropathy in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia 2008;51:562–6. 10.1007/s00125-007-0919-2 [DOI] [PubMed] [Google Scholar]
- 29.Holman N, Young RJ, Jeffcoate WJ. Variation in the recorded incidence of amputation of the lower limb in England. Diabetologia 2012;55:1919–25. 10.1007/s00125-012-2468-6 [DOI] [PubMed] [Google Scholar]
- 30.Coppini DV, Wellmer A, Weng C et al. . The natural history of diabetic peripheral neuropathy determined by a 12 year prospective study using vibration perception thresholds. J Clin Neurosci 2001;8:520–4. 10.1054/jocn.2001.0893 [DOI] [PubMed] [Google Scholar]
- 31.Coppini DV, Spruce MC, Thomas P et al. . Established diabetic neuropathy seems irreversible despite improvements in metabolic and vascular risk markers—a retrospective case-control study in a hospital patient cohort. Diabet Med 2006;23:1016–20. 10.1111/j.1464-5491.2006.01934.x [DOI] [PubMed] [Google Scholar]
- 32.Martin CL, Albers J, Herman WH et al. . Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care 2006;29:340–4. 10.2337/diacare.29.02.06.dc05-1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiggin TD, Sullivan KA, Pop-Busui R et al. . Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes 2009;58:1634–40. 10.2337/db08-1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paisey RB, Paisey RM, Thomson MP et al. . Protection from clinical peripheral sensory neuropathy in Alström syndrome in contrast to early-onset type 2 diabetes. Diabetes Care 2009;32:462–4. 10.2337/dc08-1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2015-000163supp_table1.pdf (15KB, pdf)
bmjdrc-2015-000163supp_table2.pdf (27.1KB, pdf)
bmjdrc-2015-000163supp_table3.pdf (26.5KB, pdf)
bmjdrc-2015-000163supp_table4.pdf (32.2KB, pdf)
bmjdrc-2015-000163supp_figure.pdf (75.2KB, pdf)