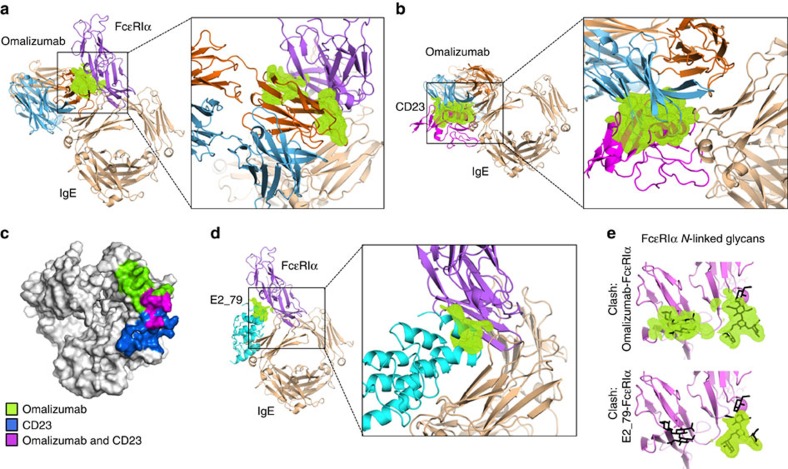
Figure 4. The structural basis of omalizumab FcɛRIα and CD23 competition.
(a) Structural alignment of complexes reveals that atomic overlap (green) between the omalizumab light chain (orange) and FcɛRIα (purple) would allow omalizumab to block IgE binding at site 2. (b) Steric conflicts (green) between the omalizumab–Fab heavy chain (blue) and CD23 (magenta) as well as direct competition for binding sites (c) appear to drive omalizumab inhibition of CD23:IgE interactions. (d) The disruptive DARPin inhibitor E2_79 (cyan) has a similar binding mode to the omalizumab Fab, yet has less atomic overlap with FcɛRIα in aligned structures of the complexes. (e) The majority of steric clashes (green) between omalizumab and FcɛRIα and E2_79 and FcɛRIα occur with N-linked glycans (black) found on FcɛRIα.