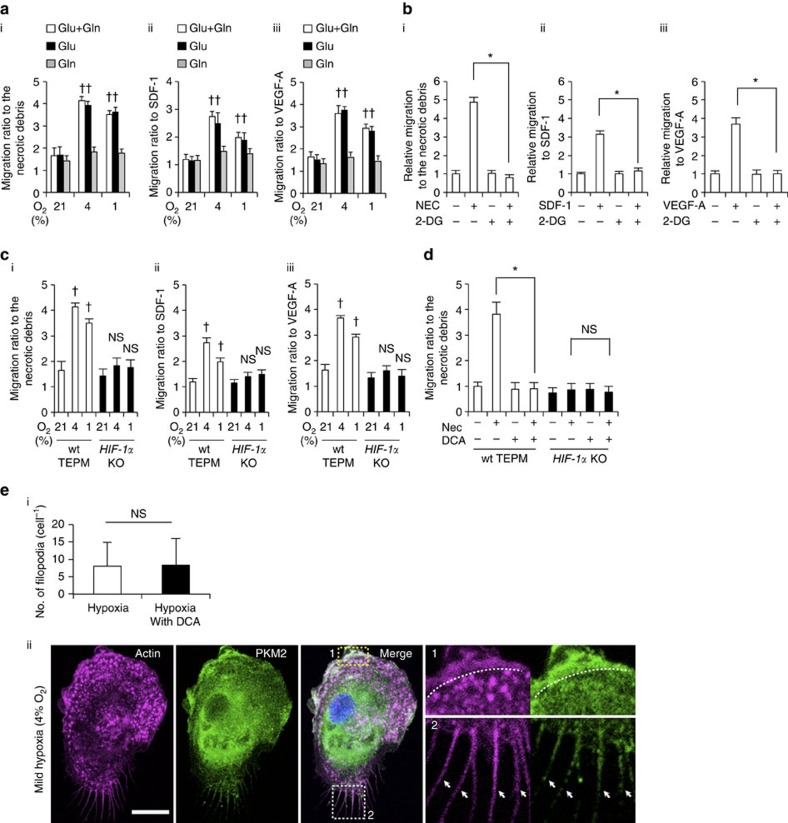
Figure 4. The role of glycolytic reprogramming in macrophage migratory capacity in vitro.
(a) (i-iii) Macrophage migration activity to various chemoattractants (the supernatant of necrotic cell debris (i), stromal cell-derived factor 1 (SDF-1; 100 ng ml−1; ii) and VEGF-A (100 ng ml−1; iii)) was measured by transwell assay in normoxia (21% O2, 4 h), mild hypoxia (4% O2, 4 h) and severe hypoxia (1% O2, 4 h). The culture mediums were supplemented with 10 mM glucose (Glu) only, 2 mM L-glutamine (Gln) only or both. The y axis represents the migration activity relative to untreated condition. n=3 animals. (b) Macrophage migration activity was measured in mild hypoxia in the presence of 2-DG (20 mM). n=3 animals. (c) Migration activity in wild-type and HIF-1α-deficient macrophages was measured in the same manner as 4 (a). n=3 animals per group. (d) Macrophage migration activity to the supernatant of necrotic cell debris was measured in mild hypoxia in the presence of DCA (10 mM). n=3 animals per group. (e) (i) The number of filopodia in TEPMs is shown. TEPMs were cultured under mild hypoxia in the presence or absence of DCA (10 mM). (average with s.d. n=100 cells analysed). (ii) Representative immunocytochemical staining of Hoechst (blue), PKM2 (green) and Phalloidin (magenta) in TEPMs under mild hypoxia is shown. F-actin and PKM2 were co-localized in lamellipodia (magnified view 1, dotted line) and filopodia (magnified view 2, arrowheads). Scale bars, 10 μm. All graphs indicate average with s.d.. Student's t-test was performed to calculate P value. *P<0.05; NS, not significant; †P<0.05 versus control (21% O2); 2-DG, 2-Deoxy-D-glucose; NEC, the supernatant of necrotic cell debris.