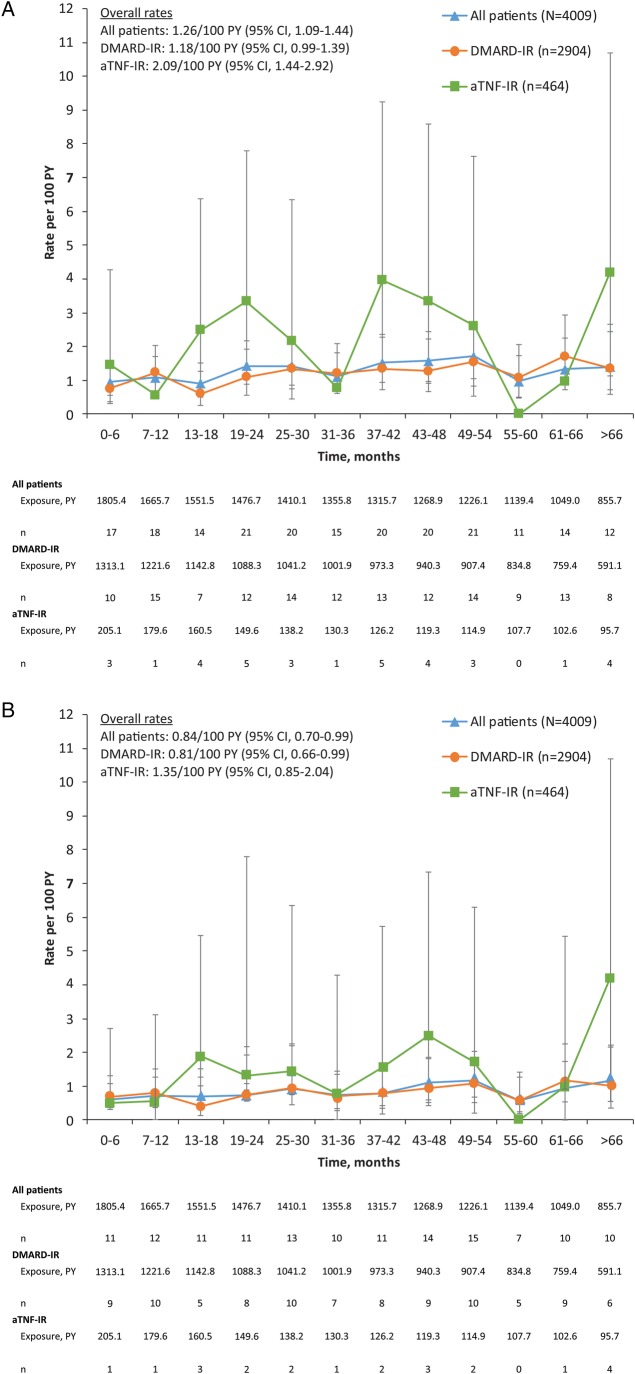
Figure 1.
Adjudicated malignancy rates including NMSC (A) and excluding NMSC (B) over time in the tocilizumab all-exposure population. Data are presented as rates/100 PY, and error bars are 95% CIs. n=total number of adverse events; multiple occurrences of the same adverse event in one patient are counted as individual events. aTNF-IR, antitumour necrosis factor-inadequate responders; DMARD-IR, disease-modifying antirheumatic drug-inadequate responders; PY, patient-years.