Case Report
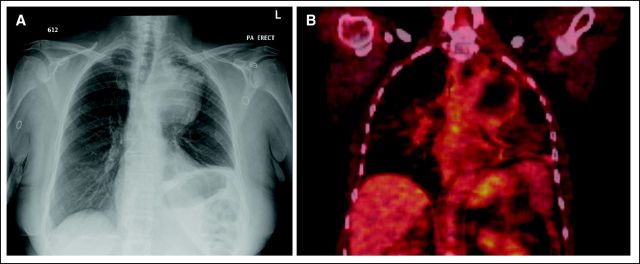
A 63-year old woman presented in November 2007 with a productive cough, dyspnea, and hoarseness. She had been previously evaluated by her primary care physician and treated with two courses of antibiotics for pneumonia without significant improvement. Her past medical history was unremarkable, with the exception of a 60 pack-year smoking history. Physical examination was significant for decreased breath sounds in the left upper hemithorax. A chest x-ray identified a 10-cm mass in the left upper lobe, with tracheal compression (Fig 1A). The patient underwent a computed tomography (CT) scan of the chest, which revealed a 10-cm necrotic mass abutting and invading into the aortopulmonary window, with associated left hilar lymphadenopathy indicative of a primary lung cancer.
Fig 1.
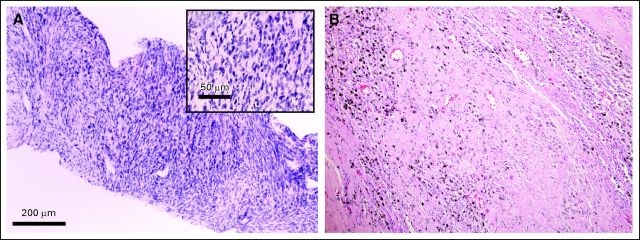
A CT-guided biopsy was performed. The tumor had the histopathological appearance of a high-grade sarcoma (Fig 2A). Immunohistochemistry was negative for cytokeratin (AE1/AE3), S-100, smooth muscle actin, desmin, epithelial membrane antigen, and CD34, but positive for vimentin, CD56, CD57, collagen 4, and neurofilament. These results were consistent with a high-grade, malignant, peripheral nerve sheath tumor. A staging positron emission tomography (PET)/CT scan showed no evidence of extrapulmonary disease (Fig 1B).
Fig 2.
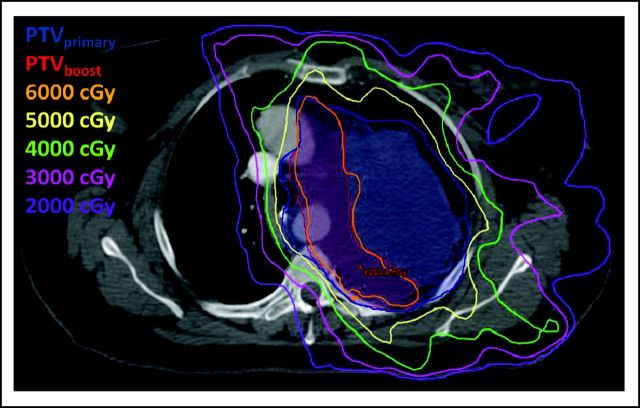
The patient was subsequently referred to our multidisciplinary sarcoma center and was evaluated by a multidisciplinary team, which included representatives from medical oncology, radiation oncology, and thoracic surgery. Given the extent of the mass, we were concerned that it would be difficult to achieve an adequate surgical margin. Therefore, we recommended preoperative concurrent chemoradiotherapy. An intensity-modulated radiation therapy (IMRT) plan was created to deliver 56 Gy to the tumor in 2-Gy daily fractions with a simultaneous integrated boost (dose painting technique) targeting the area at highest risk for a positive margin to 63 Gy in 2.25 Gy daily fractions. Daily kilovolt portal imaging and weekly cone beam CT were used for image-guided radiation therapy to confirm patient set-up. Two concurrent cycles of ifosfamide were administered on days 2 through 4 and 22 through 24 of radiation therapy at a dose of 2,500 mg/m2 for 3 days (total dose 7.5 g/m2/cycle) with hydration, antiemetic, and MESNA support. The patient's treatment course was complicated by febrile neutropenia that required hospital admission after her second cycle of chemotherapy. The final two radiation fractions were omitted as a result of acute toxicity including fatigue and dysphagia. Therefore, a total dose of 52 Gy was delivered to the entire planning target volume and 58.5 Gy to the region at highest risk for local recurrence. The primary (blue) and boost (red) planning target volumes are shown in Figure 3. The isodose lines represent the amount of radiation in centigrays delivered.
Fig 3.
A left pneumonectomy was performed 6 weeks after completion of neoadjuvant therapy. Pathology revealed extensive necrosis, with no evidence of histologically intact sarcoma (Fig 2B). Surgical margins were negative. The patient had no complications related to the procedure. Four years after her initial diagnosis, the patient has no evidence of disease and no significant late toxicity.
Discussion
Soft tissue sarcomas are characterized by a heterogeneous mix of histological subtypes and clinical presentations. The majority of soft tissue sarcomas occur in the extremities, with the next most common sites being the retroperitoneum, abdomen, and head and neck.1 Primary pulmonary sarcomas are exceedingly rare. Because pulmonary metastases from soft tissue sarcomas are seen in approximately 50% of cases and because 10% of sarcoma patients present with metastatic disease, it is important to perform an extensive evaluation for a primary site in patients who present with a pulmonary sarcoma.2 In addition, full-body imaging with a PET/CT scan may identify a primary site and provide prognostic information.3–5
The limited number of reports on primary pulmonary sarcomas suggests the overall survival rate at 5 years is approximately 38% to 44%.6–9 Factors associated with improved survival include complete surgical resection, smaller tumor size, and lower grade. Radiation therapy is an effective adjuvant treatment for soft tissue sarcomas of the extremity.10,11 In some extremity sarcomas, preoperative radiation therapy may have potential advantages over postoperative therapy, as lower doses of radiation are required to achieve a similar rate of local control.12,13 Therefore, there may be decreased rates of late toxicity. Other potential advantages of preoperative radiation therapy include a smaller treatment volume; less potential for tumor seeding during surgical resection; and better oxygenation, leading to improved radiosensitivity of the intact tumor.14 Preoperative radiation therapy increases the risk of a wound complication after surgery for soft tissue sarcoma of the lower extremity.13 However, preoperative radiation therapy to the thorax is commonly used to treat lung cancer without a substantial increase in wound complication rates.
Advances in radiation delivery, including IMRT and image guidance, have allowed for dose escalation and potentially a decreased volume of normal tissue receiving high-dose radiation.15 The use of IMRT to treat sarcomas of the extremity has been associated with excellent control rates and effective sparing of normal tissue.16 Inverse planning with IMRT and image guidance in the treatment of primary pulmonary sarcomas have the potential to limit toxicity by minimizing the high-dose region of radiation in the lung, spinal cord, and esophagus.
In this case, we applied this technology to a thoracic sarcoma, delivering an integrated boost that resulted in a 12.5% greater total dose to the margin at highest risk for recurrence. Given the higher dose per fraction, this technique results in a 17.2% increase in the biologic effective dose to the margin (assuming an alpha:beta ratio of 4). A prior report of preoperative radiation therapy in patients with retroperitoneal sarcoma has described this technique as safely escalating the radiation dose to a margin that may be challenging to resect.17 An integrated boost may be used instead of intraoperative radiation therapy (IORT) particularly when the geometry of the anticipated positive margin will be challenging to treat with IORT, such as in this case.
The use of neoadjuvant chemotherapy concurrently or interdigitated with radiation therapy for soft tissue sarcoma has been used by several groups.18–21 Ifosfamide is a nitrogen mustard alkylating agent with proven activity in soft tissue sarcoma when employed as a single agent or in combination with an anthracycline. Previous reports have suggested a benefit of single-agent ifosfamide and radiation therapy for unresectable soft tissue sarcoma.22 Furthermore, single-agent ifosfamide has been shown to be well tolerated and effective in the neoadjuvant setting.23 In a report by MacDermed et al,24 treatment-induced necrosis was associated with a lower risk of metastatic disease, suggesting both a local and systemic benefit with this approach.
Primary pulmonary sarcoma is an unusual diagnosis, with limited data available to determine the optimal treatment approach. The use of neoadjuvant chemoradiotherapy to treat primary pulmonary sarcomas has potential benefits over postoperative therapy or neoadjuvant radiation therapy alone. Advances in radiation treatment planning and delivery allow for dose escalation, particularly to margins at high risk for residual disease at surgery, and may be beneficial for patients with primary pulmonary sarcoma.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Richard F. Riedel, Merck (C) Stock Ownership: None Honoraria: Richard F. Riedel, Novartis Research Funding: Richard F. Riedel, GlaxoSmithKline, Infinity, Novartis, Ariad, Novartis, Ziopharm Expert Testimony: None Other Remuneration: None
REFERENCES
- 1.Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 2004;54:94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- 2.Coindre JM, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: A study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91:1914–1926. doi: 10.1002/1097-0142(20010515)91:10<1914::aid-cncr1214>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Schuetze SM, Rubin BP, Vernon C, et al. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer . 2005;103:339–348. doi: 10.1002/cncr.20769. [DOI] [PubMed] [Google Scholar]
- 4.Schuetze SM. Utility of positron emission tomography in sarcomas. Curr Opin Oncol. 2006;18:369–373. doi: 10.1097/01.cco.0000228744.49294.12. [DOI] [PubMed] [Google Scholar]
- 5.Quak E, van de Luijtgaarden AC, de Geus-Oei LF, et al. Clinical applications of positron emission tomography in sarcoma management. Expert Rev Anticancer Ther. 2011;11:195–204. doi: 10.1586/era.10.133. [DOI] [PubMed] [Google Scholar]
- 6.Etienne-Mastroianni B, Falchero L, Chalabreysse L, et al. Primary sarcomas of the lung: A clinicopathologic study of 12 cases. Lung Cancer. 2002;38:283–289. doi: 10.1016/s0169-5002(02)00303-3. [DOI] [PubMed] [Google Scholar]
- 7.Guccion JG, Rosen SH. Bronchopulmonary leiomyosarcoma and fibrosarcoma. Astudy of 32 cases and review of literature. Cancer. 1972;30:836–847. doi: 10.1002/1097-0142(197209)30:3<836::aid-cncr2820300335>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Janssen JP, Mulder JJ, Wagenaar SS, et al. Primary sarcoma of the lung: A clinical study with long-term follow-up. Ann Thorac Surg. 1994;58:1151–1155. doi: 10.1016/0003-4975(94)90476-6. [DOI] [PubMed] [Google Scholar]
- 9.Keel SB, Bacha E, Mark EJ, et al. Primary pulmonary sarcoma: A clinicopathologic study of 26 cases. Mod Pathol. 1999;12:1124–1131. [PubMed] [Google Scholar]
- 10.Alektiar KM, Velasco J, Zelefsky MJ, et al. Adjuvant radiotherapy for margin-positive high-grade soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys. 2000;48:1051–1058. doi: 10.1016/s0360-3016(00)00753-7. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: Prospective randomized evaluations of (1) limb-sparing surgery plus radiation-therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis AM, O'Sullivan B, Bell RS, et al. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. J Clin Oncol. 2002;20:4472–4477. doi: 10.1200/JCO.2002.03.084. [DOI] [PubMed] [Google Scholar]
- 13.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 14.Pisters PW, O'Sullivan B, Maki RG. Evidence-based recommendations for local therapy for soft tissue sarcomas. J Clin Oncol. 2007;25:1003–1008. doi: 10.1200/JCO.2006.09.8525. [DOI] [PubMed] [Google Scholar]
- 15.DeLaney TF, Trofimov AV, Engelsman M, et al. Advanced-technology radiation therapy in the management of bone and soft tissue sarcomas. Cancer Control. 2005;12:27–35. doi: 10.1177/107327480501200104. [DOI] [PubMed] [Google Scholar]
- 16.Alektiar KM, Brennan MF, Healey JH, et al. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol. 2008;26:3440–3444. doi: 10.1200/JCO.2008.16.6249. [DOI] [PubMed] [Google Scholar]
- 17.Tzeng CW, Fiveash JB, Popple RA, et al. Preoperative radiation therapy with selective dose escalation to the margin at risk for retroperitoneal sarcoma. Cancer. 2006;107:371–379. doi: 10.1002/cncr.22005. [DOI] [PubMed] [Google Scholar]
- 18.Brandts CH, Schulz C, Willich N, et al. Adjuvant therapy for resectable high-risk soft tissue sarcoma: Feasibility and efficacy of a sandwich chemoradiotherapy strategy. Cancer Chemother Pharmacol. 2012;69:613–620. doi: 10.1007/s00280-011-1731-8. [DOI] [PubMed] [Google Scholar]
- 19.DeLaney TF, Spiro IJ, Suit HD, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;56:1117–1127. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 20.Mullen JT, Kobayashi W, Wang JJ, et al. Long-term follow-up of patients treated with neoadjuvant chemotherapy and radiotherapy for large, extremity soft tissue sarcomas. Cancer. 2012;118:3758–3765. doi: 10.1002/cncr.26696. [DOI] [PubMed] [Google Scholar]
- 21.Ryan CW, Montag AG, Hosenpud JR, et al. Histologic response of dose-intense chemotherapy with preoperative hypofractionated radiotherapy for patients with high-risk soft tissue sarcomas. Cancer. 2008;112:2432–2439. doi: 10.1002/cncr.23478. [DOI] [PubMed] [Google Scholar]
- 22.Eckert F, Matuschek C, Mueller AC, et al. Definitive radiotherapy and single-agent radiosensitizing ifosfamide in patients with localized, irresectable soft tissue sarcoma: A retrospective analysis. Radiat Oncol. 2010;5:55. doi: 10.1186/1748-717X-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gronchi A, De Paoli A, Dani C, et al. Preoperative chemoradiation therapy for localized retroperitoneal soft tissue sarcoma (RSTS): A phase II study from the Italian Sarcoma Group. J Clin Oncol. 2011;29:609s. (abstr 10020) [Google Scholar]
- 24.MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft-tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147–1153. doi: 10.1016/j.ijrobp.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]