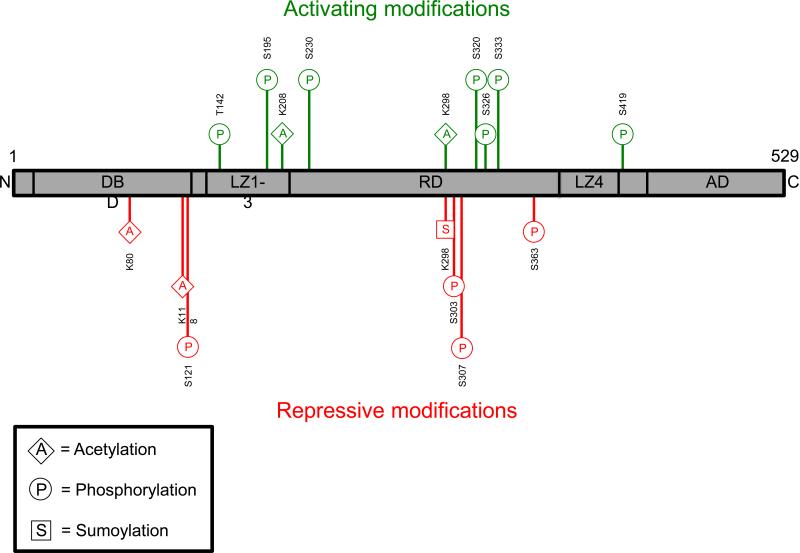
Figure 1. Heat Shock Factor 1 Post-translational Modifications and Activation Lifecycle.
1A: Heat Shock Factor 1 (HSF1) activating (green) and repressive (red) post-translational modifications (PTMs) are shown above. The bottom left box displays a PTM abbreviation key. Amino acids - K, lysine; S, serine; T, threonine. AD, activation domain; C, c-terminus; DBD, DNA-binding domain; LZ, leucine zipper domain; N, n-terminus; RD, regulatory domain.
1B: The Heat Shock Factor 1 (HSF1) activation and attenuation cycle with associated post-translational modifications (PTMs) is shown above. HSF1 forms a constitutively inactive heterotetramer with Heat Shock Protein (HSP) 40, 70, and 90. Serine (S) 121, S303, S307, and S363 phosphorylation aid in heterotetramer maintenance. (1) Upon heat shock or proteotoxic stress, the heterotetramer dissociates and S333 phosphorylation has been linked to dissociation of the repressive HSF1-HSP90 interaction. HSF1 trimerizes and translocates to the nucleus, though which occurs first has not yet been resolved. Here we show trimerization occurring first. S195 phosphorylation occurs concurrently with trimerization but this event effects transactivation downstream and not trimerization. (2) Nuclear localization is positively regulated by S320 and S419 phosphorylation. (3) After trimerization and translocation, HSF1 trimers bind to the Heat Shock Element (HSE) on HSP promoter regions. Binding is followed by transactivation. Binding is positively regulated by T142 and S320 phosphorylation and transactivation is regulated by T142, S230, S320, and S326 phosphorylation, and Lysine (K) 298 sumoylation. In addition, stabilizing acetylation events have been shown at K208 and K298. Notably, stabilizing acetylation is delayed upon transactivation and may proceed even after attenuation has begun. (4) Attenuation is initiated by newly translated HSPs, which bind to HSF1 to block HSE binding and transactivation as part of a regulatory feedback loop. K298 sumoylation and S363 phosphorylation are associated with transactivation repression. Furthermore, K80 and K118 acetylation destabilizes HSE binding. In addition, S303 and S307 phosphorylation are involved in 14-3-3ε binding to HSF1, which helps facilitate its nuclear export. (5) Upon export, HSF1 either returns to its cytoplasmic inactive state or is degraded. A, acetylation; P, phosphorylation; S, sumoylation.