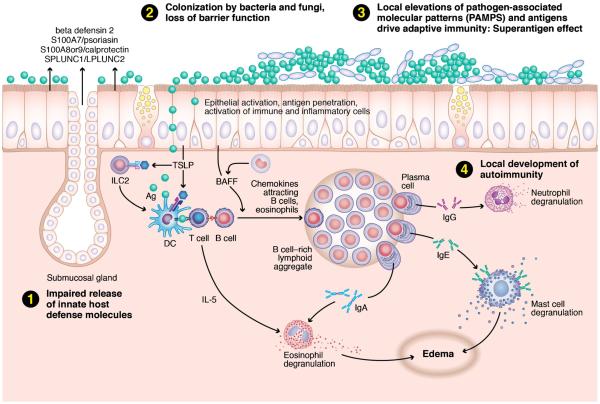
Fig. 1.
The “immune barrier hypothesis” posits that the chronic inflammation associated with CRS is linked to a defective mechanical and innate immune barrier. This deficit leads to a compensatory innate effector cell recruitment and activation, coupled with a robust adaptive immune response. The nature of this compensatory response likely varies among CRS phenotypes. Describes the proposed pathway for a typical case of Caucasian nasal polyposis. Reduced host defense molecules (1) lead to an abnormal microbiome (2), likely in biofilm format. Impaired mucociliary flow and a porous mechanical barrier lead to increased access of PAMPS stimulating a compensatory response (3). In Caucasian polypoid CRS, the cytokines BAFF and TSLP have been implicated in the observed innate and adaptive response. TSLP acts on type 2 innate lymphocytes (ILC2s) to release the type 2 cytokines IL-5 and IL-13. TSLP also acts on dendritic cells, fostering Th2 lymphocyte differentiation with an amplified type 2 cytokine response. Superantigens, if present, will accentuate the T cell response expressed in the tissue; in this case, the response is skewed to Th2. BAFF drives a strong local B cell immunoglobulin response, including autoreactive antibodies in severe cases (4). Collectively, this results in the recruitment and activation of innate effector cells that possess Fc receptors for the locally produced immunoglobulins. In the presence of large amounts of antigen that penetrate the weakened barrier, this should lead to widespread effector cell degranulation and tissue damage