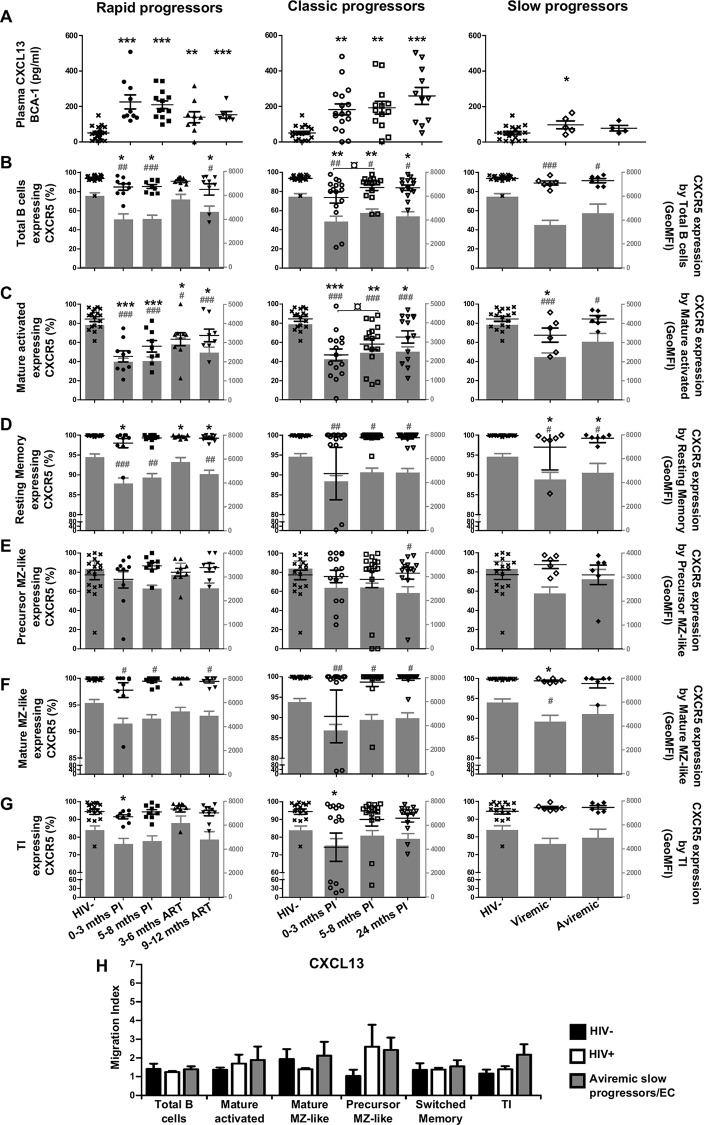
Fig 2. Analysis of plasma CXCL13 levels, CXCR5 expression and migratory potential by blood B-cells of HIV-infected individuals.
(A) Plasma concentrations (pg/ml) of CXCL13 in rapid (left panel), classic (middle panel) and slow progressors (right panel). The same HIV-negative values are used as a control for all three panels. Frequencies of B-cells expressing CXCR5 (left y axis) and levels of CXCR5 surface expression (geometric mean fluorescence intensity—geoMFI) (right y axis) by (B) total, (C) mature activated, (D) resting switched memory, (E) ‘precursor’ marginal zone (MZ)-like, (F) ‘mature’ MZ-like and (G) transitional immature (TI) B-cells of rapid (left panel), classic (middle panel) and slow progressors (right panel). The same HIV-negative values are used as a control for all three panels. Data are expressed as percentages of CXCR5 expressing-cells and intensity of surface expression within each B-cell population. (H) In vitro migration index of total, mature activated, mature MZ-like, precursor MZ-like, switched resting memory and TI B-cells from the blood of classic progressors (5–8 months PI) (n = 6), aviremic slow progressors/elite controllers (EC) (n = 6) and HIV-negative individuals (n = 6) in response to 500 ng/ml CXCL13. The in vitro migration index is defined by the number of cells that have migrated in response to a given chemokine divided by the number of cells that have spontaneously migrated. Data were compared using the Wilcoxon signed rank test and the Mann-Whitney U test for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. Data shown are mean ± SEM. Significance for percentages are expressed by * p < 0.05; ** p < 0.001; *** p < 0.0001 and intensity of surface expression by # p < 0.05; ## p < 0.001; ### p < 0.0001 when compared to HIV-negative individuals. ¤ p < 0.05 for pairwise comparisons of percentages. PI, post-infection; ART, antiretroviral therapy.