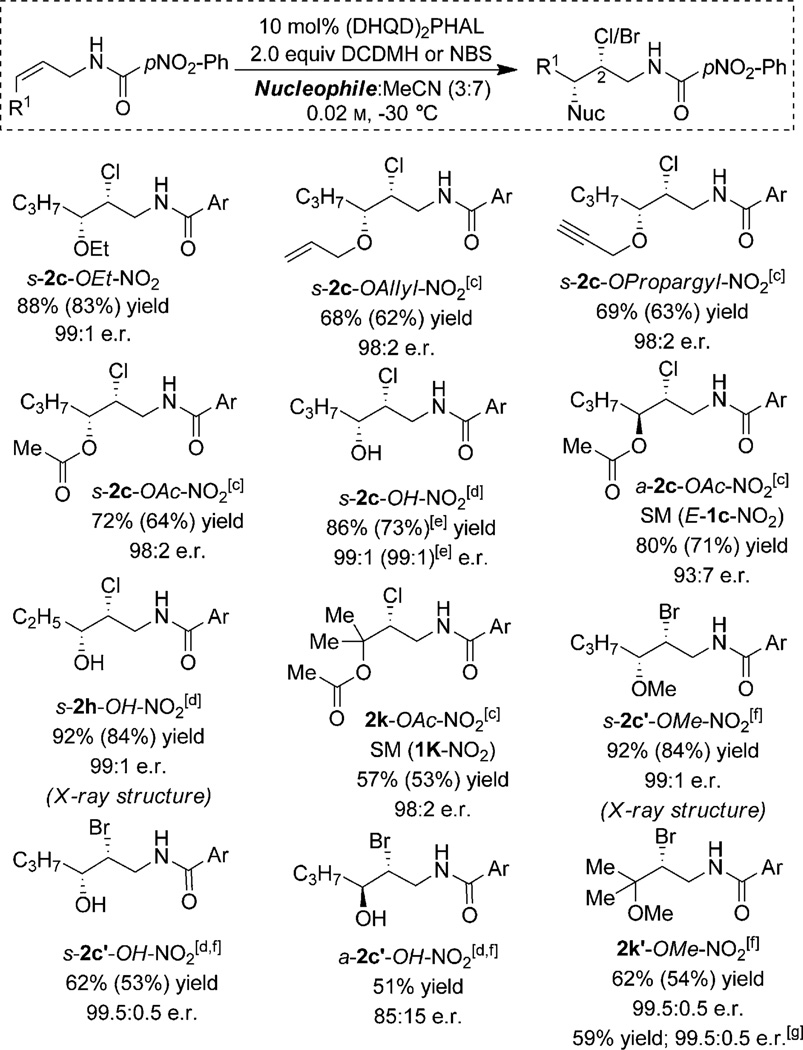
Scheme 2.
Nucleophile scope of intermolecular chlorofunctionalization.[a,b] [a] The r.r. was more than 20:1 and d.r. was more than 99:1 in all instances. [b] Yield determined by NMR with MTBE as standard. Numbers in parentheses reflect yields of isolated products on a 0.1 mmol scale. Enantioselectivity determined by chiral-phase HPLC. [c] Reaction was run under nitrogen in the presence of molecular sieves. [d] The ratio of MeCN:H2O was 9:1, reaction temperature was −10 °C. [e] The results reflect a 1 g scale reaction. The corresponding Ritter product (10%) was also isolated. [f] The prime symbol refers to Br instead of Cl in the product. [g] Results with the quasi enantiomeric (DHQ)2PHAL catalyst (ent-2k’-OMe-NO2 was the product).