Table 1.
Orienting studies for the enantioselective intermolecular chloroetherification of E-1b-(Br/OMe/NO2).
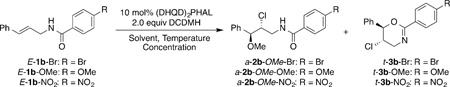 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Substrate | Solvent | Conc. [M] | Temp. [°C] | Yield [%][a] | ratio 2b:3b[b] |
d.r. (a-2b:s-2b)[b] |
e.r. (2b)[c] |
e.r. (3b)[c] |
| 1 | E-1b-Br | MeOH | 0.03 | 24 | 93 | 1.8:1 | 6.8:1 | 67:33 | 96:4 |
| 2 | E-1b-Br | MeOH | 0.03 | −30 | 94 | 1.8:1 | 6.8:1 | 73:27 | 98:2 |
| 3 | E-1b-Br | MeOH | 0.01 | −30 | 76 | 1.8:1 | 5.7:1 | 71:29 | 98:2 |
| 4[d] | E-1b-Br | MeOH/MeCN | 0.01 | −30 | 76 | 1.3:1 | 5.8:1 | 85:15 | 99:1 |
| 5[d] | E-1b-OMe | MeOH/MeCN | 0.01 | −30 | 86 | 1.3:1 | 5.0:1 | 83:17 | 97:3 |
| 6[d] | E-1b-NO2 | MeOH/MeCN | 0.01 | −30 | 85 | 1.1:1 | 3.4:1 | 92:8 | 96:4 |
Combined yield of a-2b, s-2b, and 3b as determined by NMR analysis with MTBE as added external standard.
Determined by NMR spectroscopy.
Determined by chiral-phase HPLC.
MeOH/MeCN ratio was 3:7.
