Table 2.
Reaction optimization for aliphatic substrates.[a]
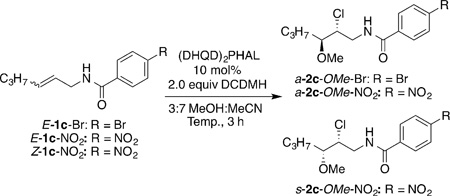 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Temp [°C] | Product | Yield [%][b] | e.r.[d] |
| 1 | E-1c-Br | −30 | a-2c-OMe-Br | 92[c] | 81:19 |
| 2 | E-1c-NO2 | −30 | a-2c-OMe-NO2 | 86 | 87:13 |
| 3 | Z-1c-NO2 | −30 | s-2c-OMe-NO2 | 87 | 99.5:0.5 |
| 4 | Z-1c-NO2 | 24 | s-2c-OMe-NO2 | 75 | 97:3 |
| 5[e] | Z-1c-NO2 | −30 | s-2c-OMe-NO2 | 79 | 97:3 |
The r.r. (defined as ratio of 2:5, see Table 4) was more than 20:1 and d.r. was more than 99:1 in all instances.
Determined by NMR using MTBE as added external standard.
5% of cyclized product was also seen by NMR.
Determined by chiral-phase HPLC.
2 mol% catalyst was used.
