Table 3.
Substrate scope of intermolecular chloroetherification.
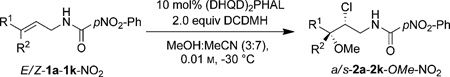 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Starting material | R1 | R2 | Product | Yield [%][a] | d.r. | r.r.[b] | e.r.[c] |
| 1 | E-1b-NO2 | Ph | H | a-2b-OMe-NO2[e] | 56 (49)[d] | 3.4:1 | >99:1 | 92:8 |
| 2 | E-1d-NO2 | pF-Ph | H | a-2d-OMe NO2[e] | 64 (58)[d] | 3.3:1 | >99:1 | 90:10 |
| 3 | E-1c-NO2 | n-C3H7 | H | a-2c-OMe NO2 | 86 (79) | >99:1 | >20:1 | 87:13 |
| 4 | E-1a-NO2 | CyHex | H | a-2a-OMe NO2 | 70 | >99:1 | >20:1 | 75:25 |
| 5 | E-1e-NO2 | BnOCH2 | H | a-2e-OMe-NO2 | 62 (57) | >99:1 | 7:1 | 89:11 |
| 6 | Z-1b-NO2 | H | Ph | s-2b-OMe-NO2 | 93 (82)[d] | 3.3:1 | >99:1 | 99.5:0.5 (90:10)[f] |
| 7 | Z-1f-NO2 | H | pMe-Ph | s-2f-OMe-NO2 | 87[d] | 1.3:1 | >99:1 | 98:2 (97:3)[f] |
| 8 | Z-1g-NO2 | H | pMeO-Ph | s-2g-OMe-NO2 | 80[d] | 1:1 | >99:1 | 99:1 (92:8)[f] |
| 9 | Z-1h-NO2 | H | C2H5 | s-2h-OMe-NO2 | 83 (77) | >99:1 | >20:1 | 98:2 |
| 10 | Z-1c-NO2 | H | C3H7 | s-2c-OMe-NO2 | 87 (79)g | >99:1 | >20:1 | 99.5:0.5 (99.5:0.5)[g] |
| 11 | Z-1i-NO2 | H | C5H11 | s-2i-OMe-NO2 | 83 (74) | >99:1 | >20:1 | 95:5 |
| 12 | Z-1e-NO2 | H | BnOCH2 | s-2e-OMe-NO2 | 60 (53) | >99:1 | 7:1 | 99.5:0.5 |
| 13 | Z-1j-NO2 | H | TBDPSOC2H4 | s-2j-OMe-NO2 | 79 | >99:1 | >20:1 | 99.5:0.5 |
| 14 | 1k-NO2 | Me | Me | 2k-OMe-NO2 | 59 (53) | na | na | 99:1 |
| 15[h] | E-1b-NO2 | Ph | H | ent-a-2b-OMe-NO2[e] | 42[d] | 6.2:1 | >99:1 | 75:25 |
| 16[h] | Z-1f-NO2 | H | pMe-Ph | ent-s-2f-OMe-NO2 | 82 | 1.2:1 | >99:1 | 99.5:0.5 (96:4)[f] |
| 17[h] | Z-1c-NO2 | H | C3H7 | ent-s-2c-OMe-NO2 | 69 | >99:1 | >20:1 | 95:5 |
Yield determined by NMR with MTBE as standard. Numbers in parentheses reflect yields of isolated product on a 0.1 mmol scale.
r.r. (regioselectivity) is defined as ratio of 2:5, see Table 4.
Enantioselectivity determined by chiral-phase HPLC.
Combined yield of diastereomers, Li2CO3 (15 equiv) was used as additive.
Mass balance was the cyclized product;
The e.r. values are for the minor diastereomer.
The result in parenthesis is from a 1 g reaction scale, illustrating the scalability of the method. The corresponding Ritter product[13] (6%) was also isolated.
The reactions in entries 15–17 were performed with the quasi enantiomeric (DHQ)2PHAL catalyst, yielding the enantiomeric product in each case.
