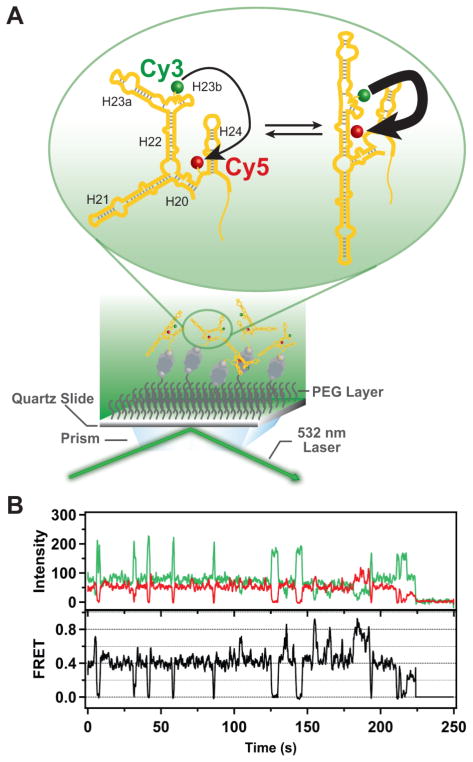
Figure 3.
Schematic illustration of the single-molecule fluorescence measurements and representative experimental data. (A) RNA labeled with Cy3 (donor) and Cy5 (acceptor) probes is immobilized on a quartz surface by biotin-streptavidin attachment (biotin is shown as a gray sphere and streptavidin as a dark grey rectangle with a biotin binding site in each corner). The surface is passivated with polyethylene glycol (PEG) molecules (wavy lines) to suppress non-specific adsorption of proteins. Upon optical excitation of the Cy3 dye by the evanescent field of the totally internally reflected green laser beam, FRET can occur from Cy3 to Cy5 (indicated by the arrows). The RNA is depicted as exchanging between two distinct conformations, with corresponding differences in FRET efficiency (indicated by the weights of the arrows). (B) Representative time traces of the Cy3 (green) and Cy5 (red) emission from a single RNA molecule immobilized on the surface. Anti-correlated fluctuations of the donor and acceptor emission are evident, indicating that FRET is occurring. The corresponding FRET intensity trajectory is shown by the black trace. In the example shown, the protein S15 is also present. A single-step donor photobleaching event is observed at ~225 s, resulting in the abrupt loss of both donor and acceptor emission.