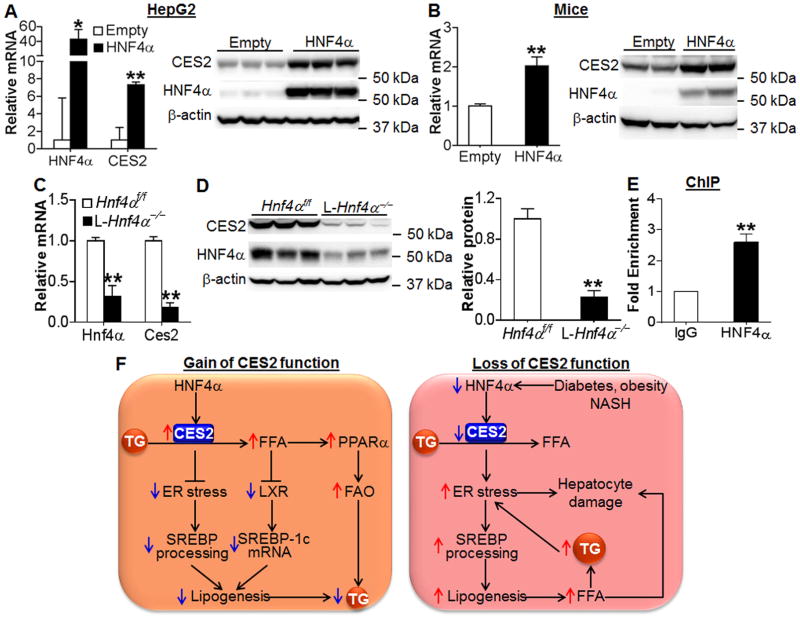
Figure 7. Hepatic CES2 is regulated by HNF4α.
(A) HepG2 cells were infected with Ad-Empty or Ad-HNF4α. After 48 h, mRNA (left panel) and protein (right panel) levels were determined (n=3). (B) C57BL/6J mice were injected i.v. with Ad-Empty or Ad-HNF4α (n=6 per group). After 7 days, Ces2 mRNA (left panel) and protein (right panel) levels were determined. (C and D) Hepatic mRNA (C) and protein (D, left panel) levels were determined and relative CES2 protein level quantified (D, right panel) in liver-specific Hnf4α−/− mice (L-Hnf4α−/−) and their control mice (Hnf4αf/f) (n=5). (E) ChIP assays were performed using liver lystates (n=4). (F) A central role for hepatic CES2 in regulating TG homeostasis. CES2 is a TG hydrolase and is regulated by HNF4α. CES2 hydrolyzes TG to release FFA, thus reducing ER stress, inhibiting LXR activity and augmenting PPARα activity. As a result, SREBP transcription/processing is repressed and FAO is increased, leading to reduced lipogenesis and TG levels in the liver. Under the conditions of metabolic stress (diabetes, obesity and NASH), hepatic HNF4α and CES2 expression is reduced, leading to increased TG accumulation and ER stress. The increased ER stress can cause hepatocyte damage and increased SREBP processing, resulting in elevated lipogenesis and FFA levels. Elevated FFAs contribute to hepatocyte damage. Increased TG levels further aggravate ER stress. *P<0.05, **P<0.01