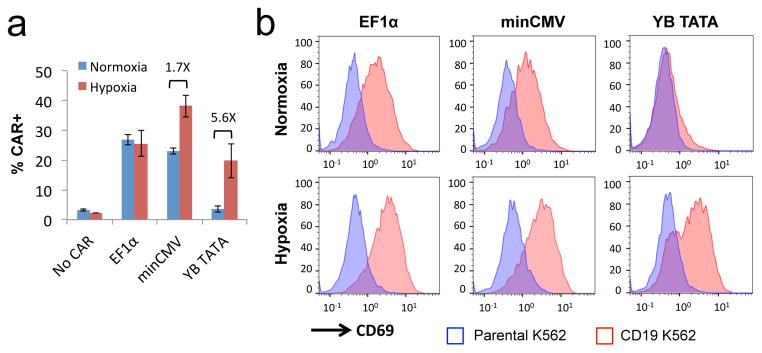
Figure 7.
Proper selection of core promoters enables restriction of antigen-stimulated T-cell activation specifically to hypoxic environments. Jurkat cells were transiently transfected with plasmids encoding a FLAG-tagged CD19 CAR expressed from a constitutive EF1α promoter or hypoxia-inducible promoters featuring either minCMV or YB_TATA as the core promoter. (a) CAR surface expression levels in transfected cells as detected by anti-FLAG antibody staining. Values shown are the means of triplicates with error bars indicating ± 1 s.d. Numbers in the plot indicate fold-induction for minCMV and YB_TATA samples. (b) Jurkat cells were cultured under normoxia for 5 hours post transfection, and then co-incubated with either parental (CD19−) or CD19+ K562 target cells for an additional 24 hours under either normoxic or hypoxic conditions. Expression of the T-cell activation marker CD69 was determined by surface antibody staining. Transfected cells were gated by dsRed+ expression prior to quantification of FLAG or CD69 staining. Data shown in (b) are representative of three independent experiments.