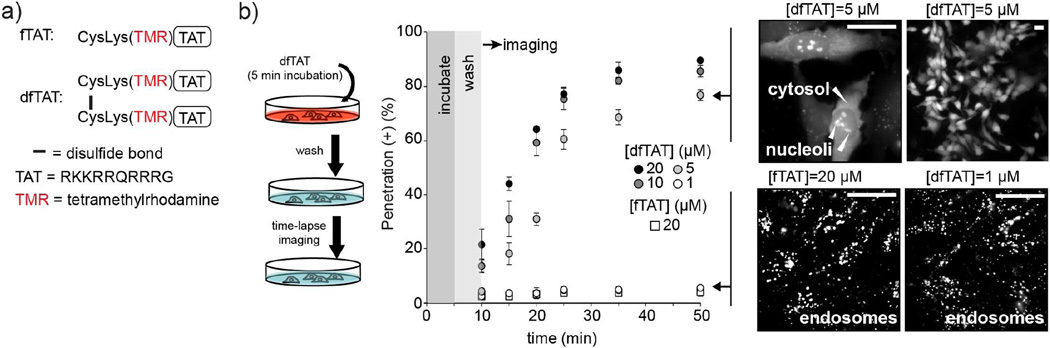
Figure 1.
dfTAT penetrates cells more efficiently than its monomeric counterpart fTAT. a) Peptide constructs. fTAT is a fluorescently-labeled analog of the prototypical CPP TAT. dfTAT is its disulfide bonded dimer (Erazo-Oliveras et al., 2014) b) Pulse chase experiment of cytosolic penetration. Cells are incubated with dfTAT at various concentrations (1, 5, 10 and 20 μM) for 5 min and washed with heparin to remove extracellular peptide. Fluorescence microscopy is then used to image cells at successive time points. Cells progress from a punctate fluorescence distribution to a cytosolic distribution when incubated with 5, 10, or 20 μM dfTAT. The fluorescence signal, presumably obtained from the reduced monomeric peptide after cytosolic access, also stains nucleoli. Cells incubated with 1 μM dfTAT or 20 μM fTAT display a punctate distribution throughout the experiment. Exclusion of the SYTOX Blue nuclear stain is used to confirm that the plasma membrane of the cells imaged is not compromised (not shown). The percentage of cells positive for cytosolic penetration, e.i. showing nucleoli staining while excluding SYTOX Blue, is reported as the mean of triplicate experiments for each conditions and the corresponding s.d. (>500 cells are imaged for each data point). Scale bars: 20 μm