Figure 1. Osmotic shock contributes a second signal required for Ca2+ dependent cPla2 activation in live zebrafish.
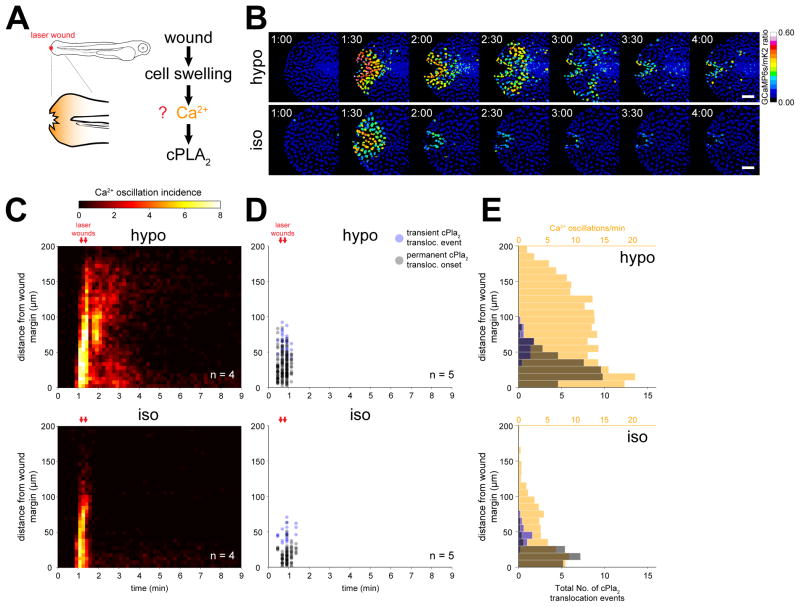
(A) Left, scheme of experimental setup. Right, working hypothesis for cell swelling-induced pathway of cPLA2 activation.
(B) Ratiometric imaging of Ca2+ transients evoked by laser-induced tail fin injury of zebrafish larvae as a function of environmental fluid osmolarity (see also Movie S1, Figure S1.). Larvae are either immersed in hypotonic solution that mimics the osmolarity of their natural habitat (“hypo”, top), or isotonic solution that mimics the osmolarity of their interstitial fluid (“iso”, bottom) to prevent cell swelling after injury. Nuclear GCaMP6s signals are normalized by nuclear mKate2 (mK2) fluorescence using the transgenic Ca2+ reporter larvae Tg(hsp70l:GCaMP6s-NLS-P2A-mK2-NLS). UV-laser wounding was performed at ~1 min. Scale bars, 50 μm.
(C) Averaged spatiotemporal profile of Ca2+ signal oscillation frequency of the indicated number of transgenic Ca2+ ratio-reporter larvae, wounded at the indicated times (red arrows) under hypotonic (top) or isotonic conditions (bottom).
(D) Spatiotemporal plot of permanent and transient cPla2-mK2 INM-translocation events induced by wounding under hypotonic (top) and isotonic conditions (bottom) in the indicated number of Tg(hsp70l:cPla2-mK2) larvae.
(E) Average spatial distribution of cPla2-mK2 translocation events (blue – transient, gray – permanent) and Ca2+ oscillation frequency (orange) as a function of distance from the wound margin, induced by wounding larvae under hypotonic (top) or isotonic (bottom) conditions (data from experiments shown in C and D).