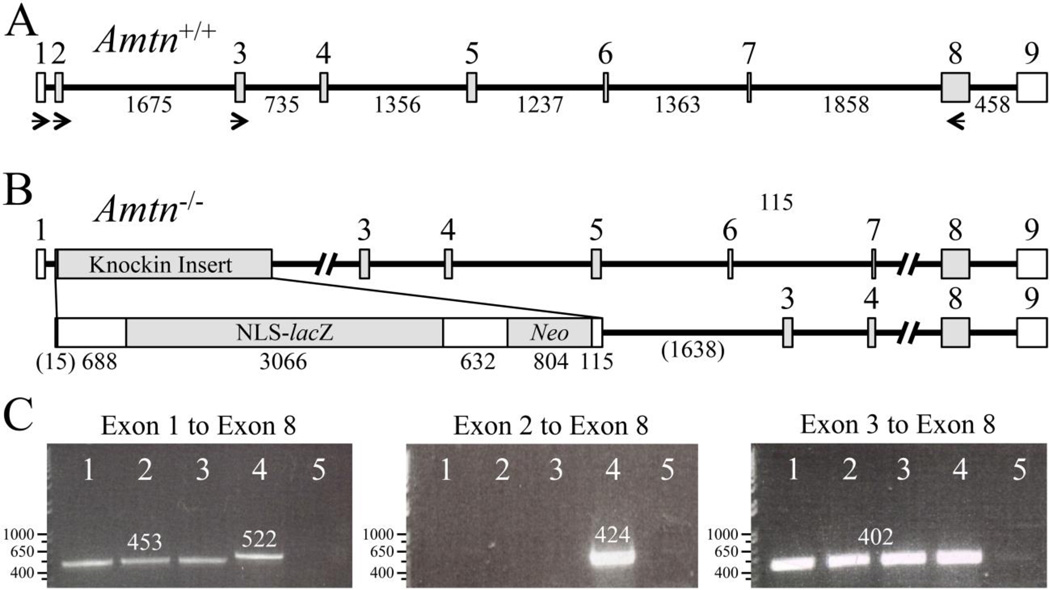
Fig. 3.
RT-PCR Assay for Amtn Expression in Amtn Null Mice. A: The wild-type Amtn gene (Amtn+/+) has 9 exons. Translation initiates early in Exon 2 and terminates early in Exon 9. The numbered boxes are exons. The horizontal lines are intron, with the number of bp below. B: The Amtn knockin gene deletes 91 bp in Amtn, starting with the Amtn translation initiation codon in Exon 2 and extending 37 bp into Intron 2. The 91 bp are replaced with 5305 bp knockin insert containing a 5’ untranslated region, NLS-lacZ code, downstream untranslated region containing a polyadenylation signal (AATAAA), neomycin phosphotransferase code, and a short 3’ untranslated region with a polyadenylation signal. C: RT-PCR using RNA template isolated from D11 (maturation stage) mandibular first molar enamel organ epithelia. Agarose gels stained with ethidium bromide; lanes 1–3 are from different Amtn−/− pups; lane 4 is wild-type (WT); and lane 5 is a negative control. The number of bp in each band is shown above the band. Left: Amtn−/− mice express an Amtn transcript that skips Exon 2 and lacks the code for translation initiation and the signal peptide. This finding explains the weakness of the lacZ reporter, despite complete loss of AMTN protein. Middle: Only WT mice express an Amtn transcript containing Exon 2. Right: Amtn+/+ and Amtn−/− mice splice the downstream Exon 3 to Exon 8 segment to remove all introns.