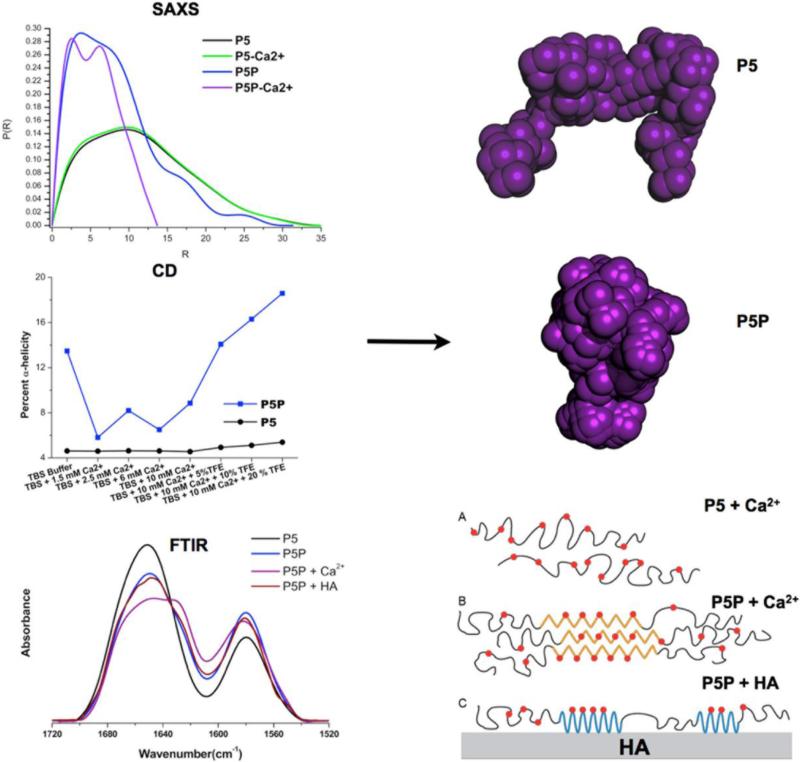
Figure 4.
In Situ Validation of Molecular Dynamics Predictions: The peptide detailed in figure 3 was studied by a variety of techniques to confirm the predictions from Molecular Dynamics. Small-angle/wide-angle X-ray scattering (SAXS/WAXS) confirmed the formation of a more structured molecule for P5P in the presence of calcium ions. This was also confirmed by circular dichroism (CD). Fourier transform infrared analysis (FTIR) combined with curve-fitting showed the peptides increased their secondary structure when bound to the HA surface; with phosphorylation (P5P) causing a shift to more helical structures. The ab initio dummy atom models (purple models) on the right were generated with the DAMMIN algorithm with SAXS data. In the lower right corner a cartoon illustrates the conformational changes suggested by these studies.