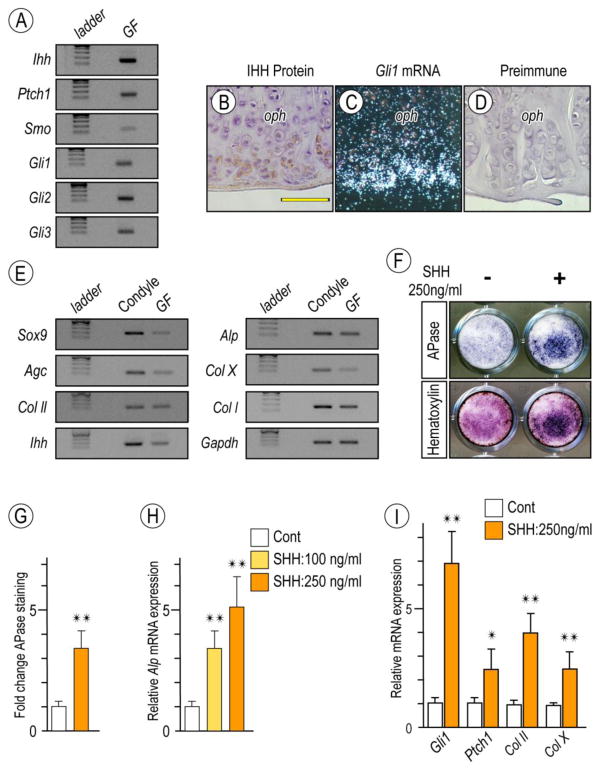
Figure 6. Detection of Hh signaling in osteoarthritic TMJs in Prg4-null glenoid fossa and Hh-induced stimulatory effect on chondrocyte maturation by primary glenoid fossa cells.
Semi-quantitative PCR analysis of expression of Ihh and its signaling associated molecules in glenoid fossae (GF)/eminences from 6-month-old Prg4-null mice (A). IHH protein expression by Immunohistochemistry (B) and Gli-1 expression by in situ hybridization (C) detected at the peripheral region of the osteophytes developing in the glenoid fossa. Sections treated with preimmune chicken IgY served as controls (D). Semi-quantitative PCR analysis of expression of chondrocyte markers in mandibular chondyle and glenoid fossa (GF) (E). Primary gleniod fossa cells were cultured on 24-well plates and stimulated with rhSHH protein (250ng/ml) for 9 days, fixed, and processed for Alkaline phosphatase activity (F; upper panel). Cultures were also counterstained with Hematoxylin to reveal the presence of cells in cultures (F; lower panel). Integrated density of Alkaline phosphatase (APase)-stained cultures was measured by ImageJ (G; n=3, *p <0.05). Untreated cultures served as reference for comparison between groups. The data are expressed in arbitrary units. Histograms depicting rhSHH-dose dependent activation of Alkaline phosphatase (Alp) mRNA (H) and increased Hh-transcriptional targets and chondrocyte maturation marker (I) in day 9 primary gleniod fossa cell culture compared to controls (*p <0.05, **p <0.02). Scale bars: 55 μm in B for B–D. oph, osteophyte.