Abstract
The role of endoscopic thyroidectomy has shown clear cosmetic benefits in the past. In this current study of 10 patients, we have tried to highlight the importance and benefits of 3D endoscopy in the management of large size multinodular goitres (MNGs) and solitary thyroid nodules (STNs). From March 2014 to July 2014, patients having a large volume of thyroid (>70 cc for one lobe) and nodule size (>6 cm) were enrolled for this study. A total of 10 patients underwent the procedure using the Karl StorzTM 3D endoscope system. Out of the 10 patients, 9 were females and 1 was male who underwent total, subtotal, and hemithyroidectomy. Three out of 10 turned out to be malignant for whom completion thyroidectomies were done endoscopically. The average blood loss was 29.5 cc and the mean operative time was 72 min. The average thyroid specimen volume was 115.4 cc with an average nodule size of 6.7 cm. Patients were discharged on the first post-operative day except one on the second post-op day. Post-operative scar was evaluated on the 14th day. 3D endoscopic thyroidectomy is definitely a step ahead in the management of large size MNGs and STNs. It gives excellent depth perception and magnification which helps in identification and preservation of important nerves and vessels which ensures safe removal of the thyroid from its bed.
Keywords: 3D endoscopy, Large size, MNGs, Endoscopic thyroidectomy
Introduction
With emerging technologies and instruments in minimal access surgery, performing complex or difficult to approach procedures has become much easier. Endoscopic thyroidectomy was first described in 2001 by Miccoli (MIVAT) [1]. After a first scepticism about the procedure by some surgeons, now, endoscopic thyroidectomy represents the first choice in many centres treating thyroid disease. It is currently performed using two-dimensional (2D) 30°, 5-mm or 0°, 10-mm endoscopes that lack in stereoscopic vision and depth of field. The recently introduced 3D endoscopes seem to overcome these limits in various surgical fields. In continuation of our series and technique of endoscopic thyroidectomy, we now present a case series of 10 patients operated endoscopically for large solitary thyroid nodule and multinodular goitres [2].
Material and Methods
From March 2014 to July 2014, patients having a large volume of thyroid (>70 cc for one lobe) and nodule size (> 6 cm) were enrolled for this study. Informed consent was obtained from all individual participants included in the study. A total of 10 patients underwent the procedure using the Karl StorzTM 3D endoscope system which had a 0° video laparoscope with two distal sensors for the optimal stereoscopic image in high-definition and DVI-D output for direct 1080p, 50/60 Hz transmission to the 3D monitor. Previously irradiated neck was the only exclusion criterion in our study. After getting approval from the ethical committee, pre-operative workup was done including FNAC of the lesion and USG neck.
The surgical instruments required include one 11-mm trocar and two 5.5-mm trocars, one 10-mm Karl StorzTM 3D endoscope, harmonic scalpel AceTM, 5-mm dissectors, scissors, an aspiration cannula, 5-mm clip applicator, and hemostats.
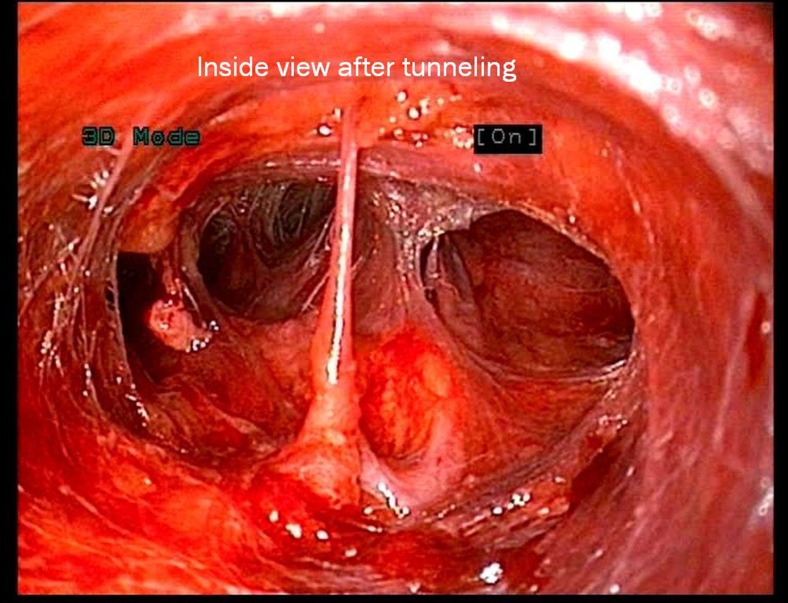
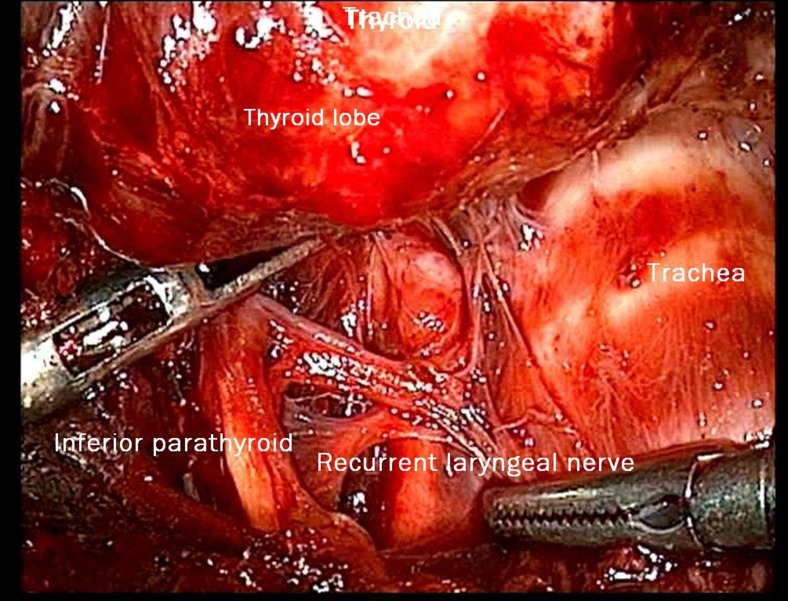
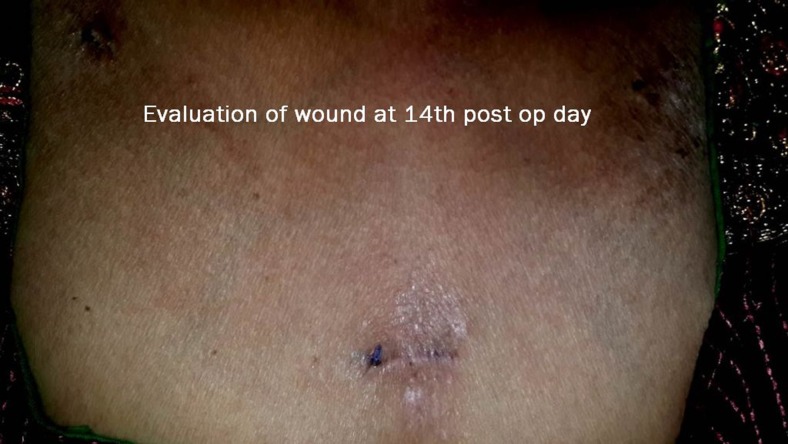
As mentioned in our earlier publication, we used the same anterior approach by placing the patient in supine position with the neck extended. Using long haemostatic forceps, a subplatysmal plane was created from a 1.5-cm transverse incision 4 cm below sternal notch and CO2 insufflation done keeping pressure at 8–10 mmHg. The subplatysmal plane was further created bluntly with the tip of the endoscope and two 5-mm ports inserted under vision bilaterally just below the clavicle in the mid-clavicular line (Fig. 1). After, the harmonic scalpel Ace was inserted from one of the 5-mm ports for sharp dissection of the subplatysmal strands, especially in the midline where the platysma is deficient. The subplatysmal plane was further developed up to the hyoid bone superiorly. The investing layer of the deep cervical fascia was incised with harmonic shears and the thyroid gland delivered in operating space after separating strap muscles laterally. Dissection begun with taking down of the inferior pole vessels close to the thyroid using the harmonic scalpel preserving supply to bilateral parathyroids and avoiding injury to the recurrent laryngeal nerve on both sides (Fig. 2). A constant traction was maintained on the thyroid lobe medially and the lobe was dissected from the lateral and posterior sides keeping the recurrent laryngeal nerve under vision. The lobe was lifted up from the trachea till the superior pole was reached. Then, the entire lobe was retracted downwards and the superior thyroid pedicle was taken using clips or coagulated using the harmonic scalpel. The superior parathyroid gland was also identified and conserved. The specimen was freed from the thyroid bed using harmonic shears. The skin incision on the sternum extended to 2.5–3.0 cm accordingly to deliver out the specimen. No drains were placed in any of the cases. Sub-cuticular absorbable Monocryl 3.0 was applied to all patients for good cosmesis. Scar evaluation was done on post-operative day 14 (Fig. 3).
Fig. 1.
Internal 3D view of tunnelling
Fig. 2.
Right parathyroid and right recurrent laryngeal nerve
Fig. 3.
Final view at the 14th post-operative day
Results
Patient’s demographics and clinical data are shown in Table 1.
Table 1.
Patient’s demographics and clinical data
| Age | Sex | Diagnosis | Largest nodule size on USG (cm) | Final HPE | Thyroid volume (cc) | Procedure | Blood loss (ml) | Operative time (min) | Post-operative stay (days) |
|---|---|---|---|---|---|---|---|---|---|
| 16 | F | STN | 6 | Colloid cyst | 72 | LT. hemithyroidectomy | 20 | 65 | 1 |
| 51 | M | MNG | 7 | MNG | 140 | Total thyroidectomy | 40 | 85 | 2 |
| 26 | F | STN | 7 | Follicular variant papillary Ca | 112 | RT. hemithyroidectomy | 25 | 70 | 1 |
| 75 | F | MNG | 8.5 | MNG | 160 | Total thyroidectomy | 35 | 80 | 1 |
| 30 | F | Follicular neoplasm | 6 | Medullary Ca | 96 | RT. hemithyroidectomy | 20 | 75 | 1 |
| 35 | F | MNG | 6.5 | MNG | 110 | Subtotal thyroidectomy | 35 | 75 | 1 |
| 48 | F | Colloid goitre | – | Hashimoto’s thyroiditis | 146 | Total thyroidectomy | 45 | 80 | 1 |
| 45 | F | MNG | 7.2 | MNG | 140 | Total thyroidectomy | 30 | 68 | 1 |
| 19 | F | Follicular neoplasm | 6.3 | Follicular variant papillary Ca | 92 | RT. hemithyroidectomy | 25 | 60 | 1 |
| 51 | F | STN | 6.4 | Colloid cyst | 86 | RT. hemithyroidectomy | 20 | 62 | 1 |
In our series of 10 patients, 9 were females and 1 was male. Hemithyroidectomy (50 %), total thyroidectomy (40 %), and subtotal thyroidectomy (10 %) were the procedures performed. In 3 out of 10 patients in whom hemithyroidectomy was performed, the final histology showed follicular variant of papillary carcinoma in two, whereas medullary Ca of the thyroid in one. Two cases which showed follicular variant of papillary Ca of the thyroid underwent endoscopic completion thyroidectomy without lymph nodal dissection within 7 days of the first procedure with excision of surgical tract used for retrieval of specimen. The third case which had suspicion of medullary Ca of the thyroid on frozen section was converted to open for completion thyroidectomy and central neck dissection. All the three completion thyroidectomies were free of tumour and lymphovascular invasion on final HPE.
Two patients with colloid cyst and three with multinodular goitre underwent hemithyroidectomy, whereas one patient underwent subtotal thyroidectomy for multinodular goitre. One patient was of Hashimoto’s thyroiditis for which total thyroidectomy was done.
The mean age of patients was 39.6 (range 16 to 75). The mean operative time was 72 min (range 60 to 85), whereas the average blood loss was 29.5 cc. The average single nodule size was 6.77 cm (range 6–8.5 cm) and the average specimen thyroid volume was 115.4 cc (largest being 160 cc).
There were no intra-operative and post-operative complications except in one case (Table 2). None of the cases had recurrent laryngeal nerve palsy or post-operative tetany. There was mild subcutaneous emphysema in one case which resolved on the second post-operative day whereas ecchymosis or hypercarbia was not observed in any patient.
Table 2.
Intra-operative and post-operative complications
| Complications | Yes/no | No. of patients |
|---|---|---|
| Nerve palsy | No | – |
| Wound infection | No | – |
| Ecchymosis | No | – |
| Subcutaneous emphysema | Yes | 1 |
Nine of the 10 patients were discharged on the first post-operative day and one on second post-operative day due to mild subcutaneous emphysema. Post-operative scar evaluation was done on the 14th post-operative day.
Discussion
Endoscopic thyroidectomy was demonstrated to be a feasible and safe procedure only if selection criteria are strictly observed due to limited working space and difficult anatomy. During the last decade, indications for endoscopic thyroidectomy were revised including 3.5 cm nodules in the maximum diameter and only 25 mL thyroid volume [1, 3]. Indications also included patients with associated thyroiditis and those with intermediate-risk differentiated thyroid cancer (DTC) rather than those with low-risk DTC [3]. In our series of 10 patients, we have tried to focus on the benefits of 3D endoscopic system in overcoming these shortcomings and the ease of management of large size multinodular goitres (MNGs) and solitary thyroid nodules (STNs). The average single nodule size was 6.77 cm (range 6–8.5 cm) and the average thyroid volume was 115.4 cc (largest being 160 cc).
According to a meta-analysis reported in literature, 2D endoscopic thyroidectomy needs longer operative time to be accomplished even if it is superior in terms of immediate post-operative pain and cosmetic results [3–5]. One restriction of endoscopic or endoscope-assisted surgery is the lack of binocular or stereoscopic vision. Monocular endoscopes give a 2D image that may impair depth perception, hand-eye coordination, and size evaluation. Some studies in other fields of application demonstrated that, although not in a strictly objective way, severe mistakes made during endoscopic procedures reflect a critical misinterpretation of the video image rather than simply technical errors.
Our earlier series of endoscopic thyroidectomy was much about the technique in which 2D, 0° video laparoscope was used [2]. Whereas, in the current series of 10 patients, the Karl StorzTM 3D endoscope system was used for increased depth perception, magnification, and ease of identifying neurovascular structures. Complications such as hypoparathyroidism, ecchymosis, or vocal cord paralysis were not observed. Conversion into conventional thyroidectomy or re-operation for haemostasis was never required. However, one patient underwent open completion thyroidectomy with central lymph node dissection in view of medullary carcinoma thyroid. Hospital stay after 3D endoscopic thyroidectomy was not exceeding 24 h except one where the patient developed subcutaneous emphysema and was discharged after 48 h.
Quality of vision was considered optimal by the users except in the presence of blood in the surgical field corresponding to a darker vision on the screen, as it happens with 2D systems. Surgeons did not report any side effects such as fatigue, headache, dizziness, and eye strain during or after surgery [6–8]. Stereoscopic visualization improves depth perception, anatomical understanding, efficiency of surgical movement, and surgeon confidence. The use of 3D endoscopic stereoscopic systems would probably improve task performance, shorten operative time, and decrease error rate.
The aim of this study was to evaluate the effectiveness and feasibility of 3D endoscopic system in the management of large size MNGs and STNs which proved to be superior to 2D system in terms of better appreciation of neurovascular structure and depth perception. However, more number of cases would be necessary to demonstrate whether a statistical difference may exist between 2D and 3D in terms of complications due to the low incidence of them. Results in terms of pain and cosmetic are expected to be similar.
Conclusion
3D endoscopic thyroidectomy is definitely a step ahead in the management of large size MNGs and STNs. It gives excellent depth perception and magnification which helps in identification and preservation of important nerves and vessels which ensures safe removal of thyroid from its bed.
Acknowledgments
Conflict of Interest
The authors declare that they have no competing interests.
Abbreviations
- MNGs
Multinodular goitres
- STNs
Solitary thyroid nodules
Contributor Information
Shailesh Puntambekar, Phone: +919822023706, Email: shase63@gmail.com.
Vikrant Sharma, Email: vikrant_doc@yahoo.com.
Sanjay Kumar, Email: drsanjayk99@gmail.com.
Sainath Mitkare, Email: dr.sainath_mitkare@yahoo.com.
Geetanjali Joshi, Email: geetanjali_agarwal@hotmail.com.
Atul Dokrimare, Email: atuldok@gmail.com.
Mangesh Panse, Email: drpmangesh@yahoo.co.in.
References
- 1.Miccoli P, Berti P, Raffaelli M, Conte M, Materazzi G, Galleri D. Minimally invasive video-assisted thyroidectomy. Am J Surg. 2001;181:567–570. doi: 10.1016/S0002-9610(01)00625-0. [DOI] [PubMed] [Google Scholar]
- 2.Puntambekar SP, Palep R, Patil A, Rayate NV, Joshi SN, Agarwal G, Joshi M. Endoscopic thyroidectomy: our technique. J Minim Access Surg. 2007;3(3):91–97. doi: 10.4103/0972-9941.37191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minuto MN, Berti P, Miccoli M, Matteucci V, Moretti M, Basolo F, Miccoli P. Minimally invasive video-assisted thyroidectomy: an analysis of results and a revision of indications. Surg Endosc. 2012;26:818–822. doi: 10.1007/s00464-011-1958-9. [DOI] [PubMed] [Google Scholar]
- 4.Miccoli P, Berti P, Raffaelli M, Materazzi G, Baldacci S, Rossi G. Comparison between minimally invasive video-assisted thyroidectomy and conventional thyroidectomy: a prospective randomized trial. Surgery. 2001;130:1039–1043. doi: 10.1067/msy.2001.118264. [DOI] [PubMed] [Google Scholar]
- 5.Pons Y, Vérillaud B, Blancal JP, Sauvaget E, Cloutier T, Le Clerc N, Herman P, Kania R. Minimally invasive video-assisted thyroidectomy: learning curve in terms of mean operative time and conversion and complication rates. Head Neck. 2013;35:1078–1082. doi: 10.1002/hed.23081. [DOI] [PubMed] [Google Scholar]
- 6.Singh A, Saraiya R. Three-dimensional endoscopy in sinus surgery. Curr Opin Otolaryngol Head Neck Surg. 2013;21:3–10. doi: 10.1097/MOO.0b013e32835bf58c. [DOI] [PubMed] [Google Scholar]
- 7.Brown SM, Tabaee A, Singh A, Schwartz TH, Anand VK. Three-dimensional endoscopic sinus surgery: feasibility and technical aspects. Otolaryngol Head Neck Surg. 2008;138:400–402. doi: 10.1016/j.otohns.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Way LW, Stewart L, Gantert W, Liu K, Lee CM, Whang K, Hunter JG. Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg. 2003;237:460–469. doi: 10.1097/01.SLA.0000060680.92690.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]