Abstract
Hypertension confers increased risk for cognitive decline, dementia, and cerebrovascular disease. These associations have been attributed, in part, to cerebral hypoperfusion. Here we posit that relations of higher blood pressure to lower levels of cerebral perfusion may be potentiated by a prior head injury. Participants were 87 community-dwelling older adults -69% men, 90% white, mean age= 66.9 years, 27.6% with a history of mild traumatic brain injury (mTBI) defined as a loss of consciousness </= 30 minutes resulting from an injury to the head, and free of major medical (other than hypertension), neurological or psychiatric comorbidities. All engaged in clinical assessment of systolic and diastolic blood pressure (SBP, DBP) and single photon emission computed tomography (SPECT). Computerized coding of the SPECT images yielded relative ratios of blood flow in left and right cortical and select subcortical regions. Cerebellum served as the denominator. Sex-stratified multiple regression analyses, adjusted for age, education, race, alcohol consumption, smoking status, and depressive symptomatology, revealed significant interactions of blood pressure and head injury to cerebral blood flow in men only. Specifically, among men with a history of head injury, higher systolic blood pressure was associated with lower levels of perfusion in the left orbital (β=-3.21, p=.024) and left dorsolateral (β=-2.61, p=.042) prefrontal cortex, and left temporal cortex (β=-3.36, p=.014); higher diastolic blood pressure was marginally associated with lower levels of perfusion in the left dorsolateral prefrontal cortex (β=-2.79, p=.051). Results indicate that men with a history of head injury may be particularly vulnerable to the impact of higher blood pressure on cerebral perfusion in left anterior cortical regions, thus potentially enhancing risk for adverse brain and neurocognitive outcomes.
Keywords: Cerebral Blood Flow, Hypertension, Traumatic Brain Injury, SPECT
1. Introduction
Hypertension has numerous negative brain health associations, including increased risk of stroke1, vascular dementia2, and Alzheimer's disease.3 Higher systolic (SBP) and diastolic blood pressure (DBP) have been further linked to decreased global4 and regional cerebral blood flow (rCBF)5. In that regard, lower rCBF has been found in the prefrontal cortex, anterior cingulate cortex, occipital lobe, and right cerebral hemisphere among hypertensives.6,7 Both men5 and the elderly6,7 may be particularly vulnerable to hypertension-related hypoperfusion. An additional factor that may render the brain vulnerable to the impact of high blood pressure is a history of head injury.
Mild traumatic brain injury (mTBI) has also been associated with reductions in cerebral blood flow.8 Although prospective studies suggest that these decrements may normalize within 1 to 3 months following the injury,8 increased prospective risk for dementia may remain.9 Persistent reductions in cerebral blood flow (CBF) have been associated with more severe TBI, but the exact mechanisms are unclear.10 It is presently unknown whether a history of head injury renders the brain more vulnerable to hypertension and/or higher blood pressure, and if CBF reduction may be compounded when both are present.
Accordingly, here we explored the potential interactive relations of blood pressure and history of mTBI to rCBF. Associations were examined separately in men and women due to known sex differences in hypertension11, mTBI12, and their associations with rCBF6.
Material and Methods
Participants were 87 healthy, community-dwelling older adults (ages 54–83; 69% men; 90% white) enrolled in a parent study investigating cardiovascular risk factors, the brain, and cognitive function in older adults.13,14 Participants were recruited for the parent study by newspaper and other local advertisement, from the Geriatric Research Education and Clinical Center at the Baltimore Veterans Affairs Medical Center (B-VAMC), and by general advertisement at the B-VAMC. A subset of participants were veterans. Exclusionary criteria were history or clinical evidence of diabetes mellitus, cardiovascular disease, coronary artery bypass surgery, carotid endarterectomy, renal, hepatic, pulmonary, hematological, or neurological disease, stroke, transient ischemic attacks, epilepsy, suspected dementia (Mini Mental State Examination<24), moderate-to-severe head injury (loss of consciousness >30 min), self-reported psychiatric disorder, moderate-to-severe depressive symptoms (Beck Depression Inventory>18), heavy alcohol use (>14 drinks/week), medication having central nervous system effects, and less than 8 years of formal education. Sample characteristics are displayed in Table 1. Of note, prevalence rates for mTBI were higher in our sample than previous documented lifetime prevalence rates for both men (16.68% compared to our 26%) and women (8.55% compared to our 30%).15 This may be due to our recruitment from the local VA, thus incorporating veterans who are more likely to sustain a combat-related mTBI. Participants provided written informed consent according to the guidelines of the University of Maryland, Baltimore and University of Maryland, Baltimore County's Institutional Review Boards.
Table 1. Demographic Characteristics.
| Demographic Variable | Men (n=62) | Women (n=27) |
|---|---|---|
| Age (M, SD) | 67.21 (6.57) | 65.81 (7.15) |
| Head Injury (% with History) | 26 | 30 |
| Education (M, SD in years) | 16.40 (2.94) | 16.30 (2.63) |
| Ethnicity (% Caucasian) | 93.9 | 83.1 |
| Beck Depression Inventory (M, SD) | 3.90 (4.03) | 5.07 (4.05) |
| Weekly Alcohol Consumption (M, SD) | 3.33 (4.24) | 1.83 (2.12) |
| Smoking Status (% Smokers) | 53.4 | 52.8 |
| Systolic Blood Pressure (M, SD, Range) | 134.65 (17.19); 95-178.3 | 125.53 (18.79); 95-178.3 |
| Diastolic Blood Pressure (M, SD, Range) | 76.11 (7.95); 50-91 | 70.71 (11.62); 53-102 |
2.1 Biomedical Assessment
Participants underwent a comprehensive medical evaluation that included history, physical examination, blood chemistries, a graded exercise treadmill test, and an oral glucose tolerance test. Head injury was based on self-reported loss of consciousness (LOC; i.e., “ever been knocked out or passed out”). If the participant reported yes, the participant was further probed about the nature of the LOC and the length of time they were unconscious. If a head injury occurred with a LOC less than 30 minutes, the participant was recorded as having a history of a mTBI. Six individuals reported having had 2 prior mTBI, and an additional 2 participants reported having a third mTBI.
BP was assessed while participants were taking their routine medications. Clinical assessment of BP was performed on 2–3 occasions with patients in a seated position using an automated vital signs monitor (Dinamap Model # 1846SX, Critikon, Tampa, FL) and appropriate sized occluding cuff. The readings were averaged to yield an estimate of participants' resting systolic and diastolic BP.
Participants also reported demographic information (age, sex, race, highest level of education completed), average weekly alcohol consumption, current smoking status and history (dichotomized into ever smoked regularly vs. never smoked), and use of anti-hypertensive medications. They also completed the Beck Depression Inventory (BDI) to measure depressive symptomatology.16
2.2 Brain SPECT Imaging
Two hours following injection with 99m Technetium Bicisate (Neurolite), participants underwent brain SPECT imaging on a Picker Prism 3000 triple headed SPECT scanner with a high-resolution fan beam collimator. The acquisition matrix was 128 × 128 pixels with a 360 degree rotation (120 degrees per head). Imaging was acquired in a series of 40 stops per head at 3 degrees per step with duration of 25 seconds per step. If necessary, an X-Y SPECT motion correction algorithm was used. Reconstruction utilized a standard ramp filter, a 3D post-filer with a 0.76 multiplier, and an attenuation correction algorithm using a coefficient of 0.12 and an ellipse fit of one per file.
Computerized coding of the perfusion images was performed using the Cerebral Blood Flow (1999) program developed by Sopha Medical Vision (SMV) America (Twinsburg, OH) on a SMV PowerStation computer. Following oblique reconstruction for anatomic normalization, images were used to derive relative ratios of blood flow in regions of interest (ROIs). ROIs were predetermined by the research team using anatomic landmarks.18 These included the right and left frontal, temporal, parietal, and occipital cortex, thalamus, head of caudate, anterior cingulate cortex, and cerebellum.
Coding methods were as follows. First, inner and outer cortical ROIs were placed. The entire cortical region was then segmented into 12 equal ROIs using four transaxial brain slices (see Figure 1 for an example). Average counts were derived from those segments corresponding to left and right frontal, temporal, parietal, and occipital lobes. For example: Slice 1: top most slice of gray/white junction (frontal); Slice 2: above the atria of lateral ventricle (frontal, parietal); Slice 3: at the level of the atria of lateral ventricle (occipital); Slice 4: just below the level of atria, above tentorium (temporal, occipital)
Figure 1. Example of coding methodology (i.e., placement of inner and outer cortical structures) with the Cerebral Blood Flow program using a representative transaxial brain slice.
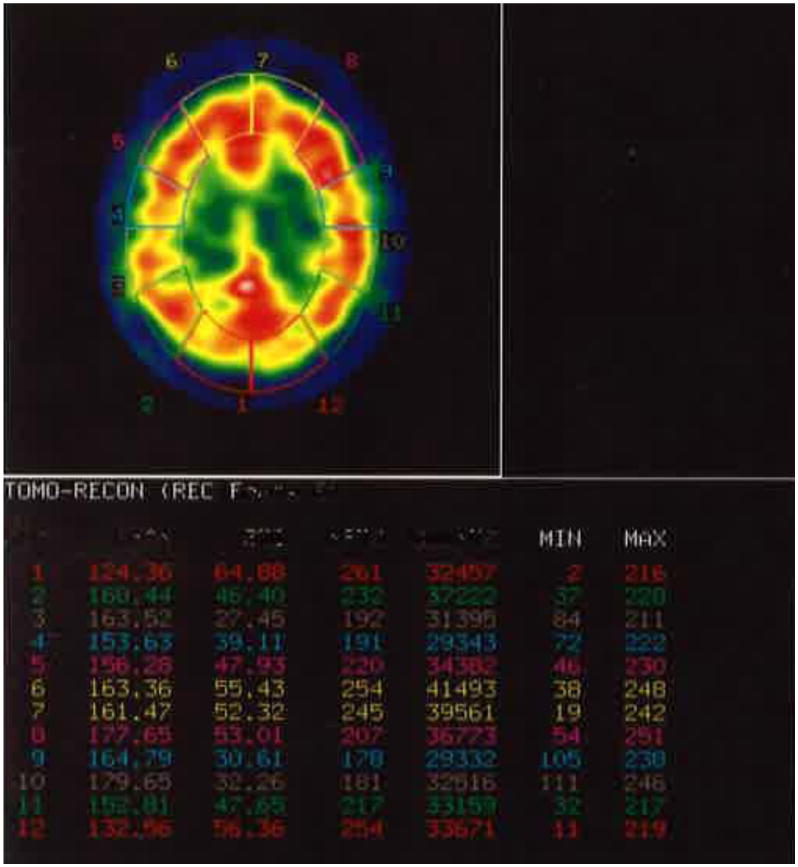
The research team next identified prefrontal subregions using anatomic landmarks19 including Fuster's20 criteria for anatomically and functionally defined prefrontal regions. These regions were drawn manually. To identify inner structures, were six ROIs were drawn manually on the left and right thalamus, head of caudate, and anterior cingulate cortex. Average counts were derived for data analytic purposes.
SPECT methodology allows assessment of relative ratios of blood flow in different brain regions. In this study, average counts for each ROI were used as a numerator to derive relative ratios of blood flow. The average counts for cerebellum was used as a standard denominator to calculate the relative uptake ratios.21 Coding was completed using nine 1.3 cm thick transaxial slices such that the top slice corresponded to the first image of the brain's gray-white junction and the ninth image corresponded to the cerebellum. All SPECT images were coded by two graduate student raters trained by a representative of SMV, and supervised by a nuclear medicine physician or physicist. To assess inter-rater reliability of the SPECT coding, iIntraclass correlations were computed, revealing coefficients greater than 0.90 across all perfusion estimates.
2.3 Data Analyses
Statistical analyses were all conducted using Statistical Package for the Social Sciences, version 22. All analyses were stratified by sex. An interaction of history of mTBI and continuous systolic BP were computed by multiplying the two variables. Multiple linear regression models were computed for each ROI as the dependent outcome, including the right and left frontal (dorsolateral, medial, orbital), temporal, parietal, and occipital cortex, thalamus, head of caudate, and anterior cingulate cortex. Systolic BP, history of mTBI, the interaction of history of mTBI and systolic BP, age, education, race, average weekly alcohol consumption, smoking status (ever/never), anti-hypertensive medication use (yes/no), and BDI score were all entered as covariates in the models. Models were then re-computed with diastolic BP. Significant interactions were then probed by computing regression line equations from the unstandardized beta coefficient for those with and without a history of mTBI.
2. Results
For men, there were significant interactions of history of mTBI and SBP for the following ROIs: left orbital prefrontal cortex (β = -3.21, p = .024), left dorsolateral prefrontal cortex (β = -2.61, p = .042), and left temporal cortex (β = -3.36, p = .014; Table 2). Also for men, there was a marginally significant interaction of history of mTBI and DBP for the ROI of left dorsolateral prefrontal cortex (β = -2.79, p = .051; Table 3). There were no significant interactions for women.
Table 2. Results from Multiple Regression Analyses of the Interaction of Systolic Blood Pressure and History of Head Injury on Regional Cerebral Blood Flow in Men*.
| Cerebral Region | p | β | Standard Error |
|---|---|---|---|
| Left Frontal | .109 | -2.101 | 1.050 |
| Right Frontal | .078 | -2.379 | 1.190 |
| Left Dorsolateral Prefrontal | .042 | -2.614 | 1.046 |
| Right Dorsolateral Prefrontal | .084 | -2.364 | 1.773 |
| Left Medial | .278 | -1.440 | 1.440 |
| Right Medial | .117 | -2.105 | 1.403 |
| Left Orbitofrontal | .024 | -3.213 | 1.607 |
| Right Orbitofrontal | .064 | -2.572 | 1.286 |
| Left Temporal | .014 | -3.364 | 1.121 |
| Right Temporal | .076 | -2.358 | 1.415 |
| Left Parietal | .401 | -1.145 | 1.718 |
| Right Parietal | .211 | -1.724 | 1.293 |
| Left Anterior Cingulate | .272 | -1.435 | 1.435 |
| Right Anterior Cingulate | .206 | -1.828 | 1.371 |
| Left Thalamus | .329 | -1.387 | 1.387 |
| Right Thalamus | .146 | -1.841 | 1.227 |
Results for women were all non-significant and therefore excluded
Table 3. Results from Multiple Regression Analyses of the Interaction of Diastolic Blood Pressure and History of Head Injury on Regional Cerebral Blood Flow in Men*.
| Cerebral Region | p | β | Standard Error |
|---|---|---|---|
| Left Frontal | .121 | -2.248 | 1.285 |
| Right Frontal | .106 | -2.401 | 1.501 |
| Left Dorsolateral Prefrontal | .051 | -2.786 | 1.238 |
| Right Dorsolateral Prefrontal | .163 | -2.118 | 1.513 |
| Left Medial | .480 | -1.042 | 1.389 |
| Right Medial | .128 | -2.205 | 1.470 |
| Left Orbitofrontal | .099 | -2.492 | 1.384 |
| Right Orbitofrontal | .062 | -2.713 | 1.357 |
| Left Temporal | .060 | -2.719 | 1.360 |
| Right Temporal | .101 | -2.278 | 1.424 |
| Left Parietal | .165 | -2.079 | 1.485 |
| Right Parietal | .180 | -2.031 | 1.451 |
| Left Anterior Cingulate | .215 | -1.780 | 1.526 |
| Right Anterior Cingulate | .236 | -1.874 | 1.406 |
| Left Thalamus | .353 | -1.411 | 1.411 |
| Right Thalamus | .221 | -1.617 | 1.213 |
Results for women were all non-significant and were therefore excluded
The significant interactions were probed by plotting simple slopes to clarify the nature of the interaction: for men only, the effects of SBP on the ROIs of the left dorsolateral prefrontal cortex, the left orbital prefrontal cortex, and the left temporal cortex were stratified by history of mTBI (Figure 2). For individuals with a history of mTBI, as SBP increased, rCBF decreased, whereas among individuals with no history of mTBI, there was no relation between SBP and rCBF. Additionally in men, the interaction of history of mTBI and DBP for the ROI of left dorsolateral prefrontal cortex (Figure 2) was also separated. For individuals with a history of mTBI, as DBP increased, the rCBF in the ROI decreased whereas in individuals with no history of mTBI, there was no relation between DBP and rCBF.
Figure 2. Interaction graphs of mTBI and BP (systolic and diastolic) with significant cerebral blood flow regions.
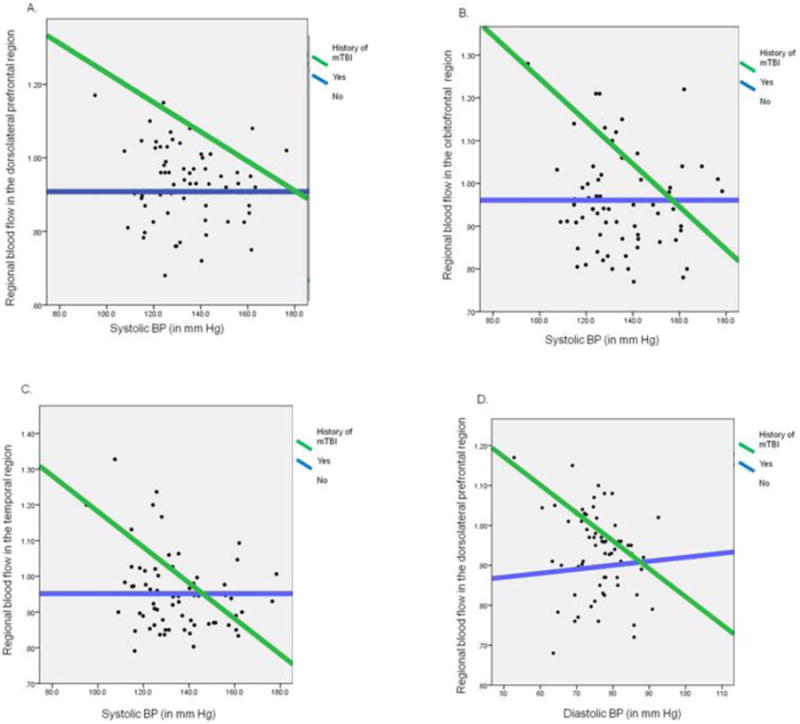
A: The interaction of mTBI history and SBP on the dorsolateral frontal blood flow for men
B: The interaction of mTBI history and SBP on the orbitofrontal blood flow for men
C: The interaction of mTBI history and SBP on the temporal blood flow for men
D: The interaction of mTBI history and DBP on the dorsolateral prefrontal blood flow for men
SBP: Systolic Blood Pressure
DBP: Diastolic Blood Pressure
BP: Blood Pressure
mTBI: Mild Traumatic Brain Injury
3. Discussion
To our knowledge, this is the first investigation to examine interactive relations of BP and mTBI history to regional cerebral perfusion. Results indicated that, in men with a history of mTBI, relative ratios of CBF in the left dorsolateral and orbital prefrontal cortex and the left temporal cortex decreased as SBP increased; CBF decreases were also noted in the left dorsolateral prefrontal cortex with DBP increases. These results suggest that men with a history of head injury may be particularly vulnerable to the impact of higher BP on cerebral perfusion in left anterior brain regions, thus potentially enhancing risk for adverse brain and neurocognitive outcomes.
Border-zone or watershed regions within the brain have been shown to be especially vulnerable to hypoperfusion and infarction.22 One of these areas is in the prefrontal cortex, located between the regions perfused by the anterior and middle cerebral arteries.23 Due to the vulnerability of this area to hypoperfusion, it is conceivable that higher blood pressure in the context of prior mTBI results in decreased circulation in the brain, affecting the dorsolateral and orbital prefrontal cortex.
Prior research has indeed indicated that the prefrontal cortex is vulnerable to the effects of hypertension alone. A reduction in white matter volume and greater white matter hyperintensities have also been found in the prefrontal cortex of hypertensives compared to controls, with no other brain area being significantly affected.24 mTBI has similarly been shown to affect the frontal lobes as well, with some studies suggesting associated executive dysfunction in the short-term25 and the long term.26 Findings from the present study suggest that the impact of both higher BP and mTBI on prefrontal cortex (in men) is synergistic, rather than additive. The temporal lobe hosts the hippocampus and amygdala, structures essential to memory and emotions.27 Interestingly, short-term memory and emotional changes28 are two of the more notable complaints of individuals that have sustained a mTBI and this association has been corroborated by formal neuropsychological assessments.25, 29 The hippocampus is also particularly vulnerable to hypoxic brain injury, with hippocampal cells being among the first to die without proper perfusion to the brain.30 Given previously noted associations of hypertension and temporal hypoperfusion5 combined with the vulnerability of the hippocampus and amygdala to mTBI, it is conceivable that the temporal lobe is particularly vulnerable to the combination of mTBI and hypertension.
While the majority of the CBF literature shows bilateral effects of different health conditions in various regions of the brain, there has also been some evidence of unilateral perfusion effects. Hypertensives have been found to have greater activation in the right hemisphere when performing well on verbal memory tasks with no activation on the left, perhaps providing indirect support of our findings of lesser perfusion in the left hemisphere of hypertensives with mTBI.31
Prior studies have also noted sex differences in the relations of various forms of brain pathology to rCBF. In that regard, increased systolic and diastolic blood pressure were associated with decreased CBF in men but not in women.5 When compared to women, men have also shown more significant reductions in rCBF in the presence of Alzheimer's disease.32 Men are also more likely to sustain a head injury33 and are at higher risk for cardiovascular diseases such as stroke34 and hypertension.35
Because our analyses were cross-sectional, it is possible that CBF changes predated the mTBI and conferred greaterer risk for head injury. For instance, mild functional and structural cerebral abnormalities such as reduced prefrontal perfusion have been noted in conditions like Attention Deficit Hyperactivity Disorder36 and substance use37. Individuals with these conditions are also more likely to sustain a mTBI38, 39. It is therefore possible that a preexisting condition may also explain the lower levels of CBF c among individuals with a mTBI.
Our findings have several potential implications. Reduced CBF may precede structural alterations in the brain which may then trigger brain pathology related to Alzheimer's disease.3 Thus, the combination of mTBI and hypertension may promote future risk for dementia or other cognitive impairments. Additionally, despite evidence that mTBI does not leave lasting structural or functional changes in the brain,8 our study may illustrate a sustained vulnerability that can compound with other negative exposures to promote poor brain health outcomes.
The current study had several limitations. First, the study sample was relatively small, especially among women with a history of mTBI, thus limiting statistical power and ability to detect smaller effect sizes. We also did not correct for Type-I error due to the exploratory nature of our study this could result in spurious effects. Additionally the generalizability of the findings is limited due to the homogenous, non-representative, convenience sample of primarily well-educated, white individuals. Also because only individuals with mild to moderate hypertension were admitted to the parent study, the BP range was truncated. Another limitation is the self-reported measure of mTBI based on LOC which could mislabel an individual if inaccurate. There was also no information collected on the nature and location of the injury, and length of time post-injury. Furthermore, the data obtained were cross-sectional, thus precluding any temporal inferences. Lastly, brain SPECT imaging is a semi-quantitative methodology that offers less spatial resolution than PET or arterial spin labeling (ASL), and has limited ability to estimate perfusion in subcortical ROIs, SPECT imaging was selected for use in the present investigation due to the unavailability of positron emission tomography at our institution at the time of study inception in 1995 (with data collected through 2008).17 Future research may benefit from the greater precision of PET or ASL, and potentially increased participation rates by using the radiation free ASL methodology.
4. Conclusions
In conclusion, the present study is the first to demonstrate interactive relations of higher blood pressure levels and mTBI to CBF in regions with known vulnerability to hypoperfusion. Men with both a history of mTBI and higher blood pressure showed decreased CBF in several left anterior cortical regions involved in executive functions, memory, and emotion. Men with a history of head injury may benefit from careful blood pressure screening and prevention of hypertension. Additional studies should be performed to further investigate the effects of more severe injuries and additional brain pathologies.
Highlights.
We examine the interaction of head injury and blood pressure on cerebral perfusion
We examined men and women separately
Higher blood pressure was associated with lower perfusion for men with head injury
Left anterior cortical regions were the most vulnerable in men only
Men with head injury may be at enhanced risk for adverse brain outcomes
Acknowledgments
Study funding: Supported by National Institutes of Health (NIH) grants R29 AG15112, 5RO1 AG015112, NIH P30-AG02874, Bristol Myers Squibb Medical Imaging, Inc., NIH K24 AG00930; a VA Merit Grant, the Department of Veterans Affairs Baltimore Geriatric Research Education and Clinical Center (GRECC), and the Geriatrics and Gerontology Education and Research Program of the University of Maryland, Baltimore.
Author Disclosure Statement: Jason Kisser reports no disclosures.
Allyssa Allen reports no disclosures.
Les Katzel reports support by the Baltimore VA Medical Center Geriatrics Research Education and Clinical Center (GRECC), National Institute on Aging (NIA) Claude D. Pepper Older Americans Independence Center NIH grants P60-AG12583, P30-AG02874 and R29 AG15112, and RO1 AG015112. He has no other disclosures or conflicts of interest.
Carrington Wendell reports no disclosures.
Eliot Siegel reports no relevant disclosures
David Lefkowtiz reports no disclosures.
Shari Waldstein reports:
• Principal Investigator:
NIA Intramural Research Program Contract: Subclinical vascular disease and change in neurocognition in the Healthy Aging in Neighborhoods of Diversity across the Lifespan (HANDLS) Study. National Institute on Aging HANDLS SCAN substudy: race, socioeconomic status, and the brain, National Institute on Aging, 1 RO1 AG034161
• Co-Investigator:
Randomized trial of exercise training on cognitive and physical function in CKD, National Institute of Diabetes and Digestive and Kidney Diseases, 1 RO1 DK090401
• Mentor/Co-Mentor:
Race, childhood social disadvantage, and the adult brain: HANDLS BRAINCHILD. National Institute on Aging., 1 KO1 AG043581
Diet quality, inflammation, race, and cognition: an analysis of HANDLS INBRE, National Institute of General Medical Sciences.
Clinical implications of sickle cell disease stigma. National Heart, Lung, and Blood Institute, 1 K07 HL108742
• Consultant:
Executive function in children with hypertension. National Heart, Lung, and Blood Institute, 1 RO1 HL098332
Aging well, sleeping efficiently. National Institute on Aging, 2 PO1 AG020677
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jason E. Kisser, Email: Jk6@umbc.edu.
Allyssa J. Allen, Email: allyssaallen@gmail.com.
Leslie I. Katzel, Email: lkatzel@grecc.umaryland.edu.
Carrington R. Wendell, Email: carringtonwendell@gmail.com.
Eliot L. Siegel, Email: esiegel@umaryland.edu.
David Lefkowitz, Email: dlefkowitz@gmail.com.
Shari R. Waldstein, Email: waldstei@umbc.edu.
References
- 1.Sacco RL. Newer risk factors for stroke. Neurology. 2001;57:S31–S34. doi: 10.1212/wnl.57.suppl_2.s31. [DOI] [PubMed] [Google Scholar]
- 2.Lis CG, Gaviria M. Vascular dementia, hypertension, and the brain. NeurolRes. 1997;19:471–480. doi: 10.1080/01616412.1997.11740844. [DOI] [PubMed] [Google Scholar]
- 3.Skoog I, Gustafson D. Update on hypertension and Alzheimer's disease. Neruol Res. 2006;28:605–611. doi: 10.1179/016164106X130506. [DOI] [PubMed] [Google Scholar]
- 4.Salerno JA, Grady C, Mentis M, Gonzalez-Aviles A, Wagner E, Schapiro MB, Rapoport SI. Brain metabolic function in older men with chronic essential hypertension. J Gerontol A Biol Med Sci. 1995;50:M147–M154. doi: 10.1093/gerona/50a.3.m147. [DOI] [PubMed] [Google Scholar]
- 5.Waldstein SR, Lefkowitz DM, Siegel EL, Rosenberger WF, Spencer RJ, Tankard CF, Manukyan Z, Gerber EJ, Katzel L. Reduced cerebral blood flow in older men with higher levels of blood pressure. J Hypertens. 2010;28:993–998. doi: 10.1097/hjh.0b013e328335c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA, Resnick SM. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;38:1766–1773. doi: 10.1161/STROKEAHA.106.477109. [DOI] [PubMed] [Google Scholar]
- 7.Jennings JR, Muldon MF, Ryan CM, Mintun MA, Meltzer CC, Townsend DW, Sutton-Tyrrell K, Shapiro AP, Manuck SB. Cerebral blood flow in hypertensive patients: An initial report of reduced and compensatory blood flow responses during performance of two cognitive tasks. Hypertension. 1998;31:1216–1222. doi: 10.1161/01.hyp.31.6.1216. [DOI] [PubMed] [Google Scholar]
- 8.Schretlen DJ, Shapiro AM. A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int Rev Psychiatry. 2003;15:341–349. doi: 10.1080/09540260310001606728. [DOI] [PubMed] [Google Scholar]
- 9.Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC. Increased risk ofdementia in patients with mild traumatic brain injury: A nationwide cohort study. PLoS One. 2013;8:e62422. doi: 10.1371/journal.pone.0062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 11.Recklehoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 12.Farace E, Alves WM. Do women fare worse: a metaanalaysis of gender differences in traumatic brain injury outcome. J Neurosurg. 2000;93:539–545. doi: 10.3171/jns.2000.93.4.0539. [DOI] [PubMed] [Google Scholar]
- 13.Waldstein SR, Katzel LI. Interactive relations of central versus total obesity and blood pressure to cognitive function. Int J Obes (Lond) 2006;30:201–207. doi: 10.1038/sj.ijo.0803114. [DOI] [PubMed] [Google Scholar]
- 14.Waldstein SR, Siegel EL, Lefkowitz D, Maier KJ, Pelletier Brown JR, Obuchowski AM, Katzel LI. Stress-induced blood pressure reactivity and silent cerebrovascular disease. Stroke. 2004;35:1294–1298. doi: 10.1161/01.STR.0000127774.43890.5b. [DOI] [PubMed] [Google Scholar]
- 15.Frost RB, Farrer TJ, Primosch M, Hedges DW. Prevalence of traumatic brain injury in the general adult population: A meta-analysis. Neuroepidemiology. 2013;40:154–159. doi: 10.1159/000343275. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 17.Wintermark M, Sesay M, Barbier E, Borbely K, Dillon WP, Eastwood JD, Glenn TC, Grandin CB, Pedraza S, Soustiel JF, Nariai T, Zaharchuk G, Caielle JM, Dousset V, Yonas H. Comparative overvier of brain perfusion imaging techniques. Stroke. 2005;36:e83–e99. doi: 10.1161/01.STR.0000177884.72657.8b. [DOI] [PubMed] [Google Scholar]
- 18.Nolte J, Angevine JB. The human brain: In photographs and diagrams. Mosby Year Book Inc.; St.Louis, MO: 1995. [Google Scholar]
- 19.Nolte J, Angevine JB. The human brain: In photographs and diagrams. St Louis: MO; Mosby-Year Book, Inc; 1995. [Google Scholar]
- 20.Fuster JM, editor. The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe. 3rd. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 21.Schmitt FA, Shih W, DeKosky ST. Neuropsychological correlates of single photon emission computed tomography (SPECT) in Alzheimer's disease. Neuropsychology. 1992;6:159–171. [Google Scholar]
- 22.Momijian-Mayor I, Baron JC. The pathophysiology of watershed infarction in internal carotid artery disease: Review of cerebral perfusion studies. Stroke. 2005;36:567–577. doi: 10.1161/01.STR.0000155727.82242.e1. [DOI] [PubMed] [Google Scholar]
- 23.Blumenfeld H. Neuroanatomy through Clinical Cases. 2nd. Sinauer Assoc.; Sunderland: 2010. [Google Scholar]
- 24.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 25.Brenner LLA. Neuropsychological and neuroimaging findings in traumatic brain injury and post-traumatic stress disorder. Dialogues Clin Neurosci. 2011;13:311–323. doi: 10.31887/DCNS.2011.13.3/lbrenner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tunvirachaisakul C, Thavichachart N, Worakul P. Executive dysfunction among mild traumatic brain injured patients in Northeastern Thailand. Asian Biomed. 2011;5:407–411. [Google Scholar]
- 27.Schoenberg MR, Scott JG, editors. The Little Black Book of Neuropsychology: A Syndrome-Based Approach. Springer; New York: 2011. [Google Scholar]
- 28.Cicerone K, Kalmar K. Persistent postconcussion syndrome - The structure of subjective complaints after mild traumatic brain injury. J Head Trauma Rehabil. 1995;10:1–17. [Google Scholar]
- 29.Mathias JL, Coats JL. Emotional and cognitive sequelae to mild traumatic brain injury. J Clin Exp Neuropsychol. 1999;21:200–215. doi: 10.1076/jcen.21.2.200.930. [DOI] [PubMed] [Google Scholar]
- 30.Cervós-Navarro J, Diemer NH. Selective vulnerability in brain hypoxia. Crit Rev Neurobiol. 1991;6:149–182. [PubMed] [Google Scholar]
- 31.Jennings JR, Muldon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, van der Veen FM, Meltzer CC. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64:1358–1365. doi: 10.1212/01.WNL.0000158283.28251.3C. [DOI] [PubMed] [Google Scholar]
- 32.Hanyu H, Shimizu S, Tanaka Y, Takasaki M, Koizumi K, Abe K. Difference in regional cerebral blood flow patterns in male versus female patients with Alzheimer disease. Am J Neuroradiol. 2004;25:1199–1204. [PMC free article] [PubMed] [Google Scholar]
- 33.Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. CDC; Atlanta: 2010. [Google Scholar]
- 34.Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 35.De la Torre JC. Alzheimer disease as a vascular disorder: Nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 36.Spalletta G, Pasini A, Pau F, Guido G, Menghini L, Caltagirone C. Prefrontal blood flow dysregulation in drug naïve ADHD children without structural abnormalities. J Neural Transm. 2001;108:1203–1216. doi: 10.1007/s007020170010. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addition: Neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keenan HT, Hall GC, Marshall SW. Early head injury and attention deficit hyperactivity disorder: Restrospective cohort study. BMJ. 2008;337:a1984. doi: 10.1136/bmj.a1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor LA, Kreutzer JS, Demm SR, Meade MA. Traumatic brain injury and substance abuse: A review and analysis of the literature. Neuropsychol Rehabil. 2003;13:165–188. doi: 10.1080/09602010244000336. [DOI] [PubMed] [Google Scholar]


