Figure 5.
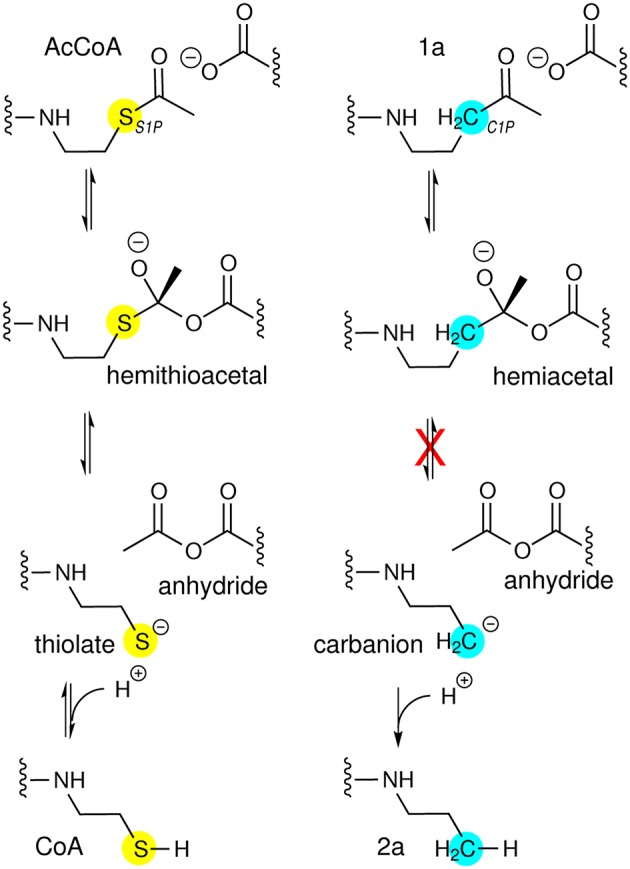
CoA analogs as probes of CoA-transferase mechanism. (Left) Reactions involving the external carbonyl of the acetylglutamyl anhydride. Thiolate protonation is possible but unlikely to be part of the normal reaction coordinate. (Right) Attempted trapping of a tetrahedral external oxyanion intermediate. The atomic substitution in 1a has comparatively small effects on electronegativity and sterics so could allow formation of a hemiacetal intermediate resembling the external oxyanion and perhaps enable insight into how its reactivity is modulated. The tetrahedral intermediate formed by nucleophilic attack by AarC on the 1a carbonyl cannot be resolved into cleavage products. The very high pKa of an unactivated alkyl methyl, perhaps 50, would preclude formation of the alkyl carbanion required, even considering the exothermic subsequent protonation.
