Figure 1.
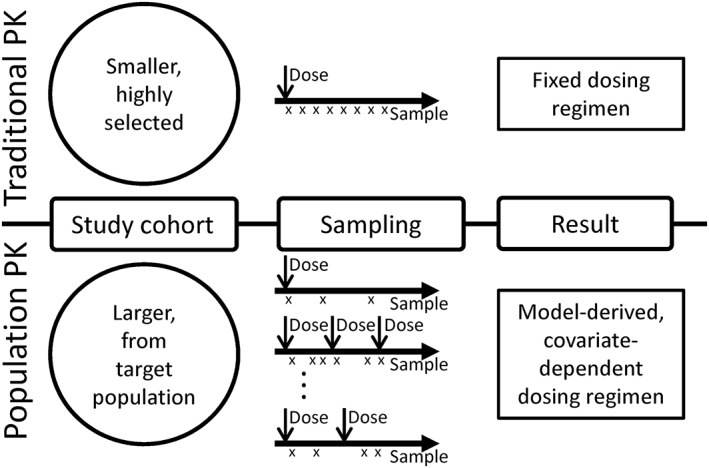
Traditional vs. population pharmacokinetic (PK) analyses. In traditional PK studies (top), small cohorts of patients, often healthy volunteers, are administered a drug. Serial samples are obtained to determine drug concentration at precise times after the dose, from which PK parameters are calculated. These parameters determine fixed dosing regimens for the study drug. In population PK studies (bottom), larger cohorts of patients are required and often include patients who are being treated with the study drug as part of clinical care. Fewer samples are obtained from each patient and are obtained at variable times after drug doses are given. These data are used to determine model based dosing, incorporating covariates that are determined to influence drug kinetics
