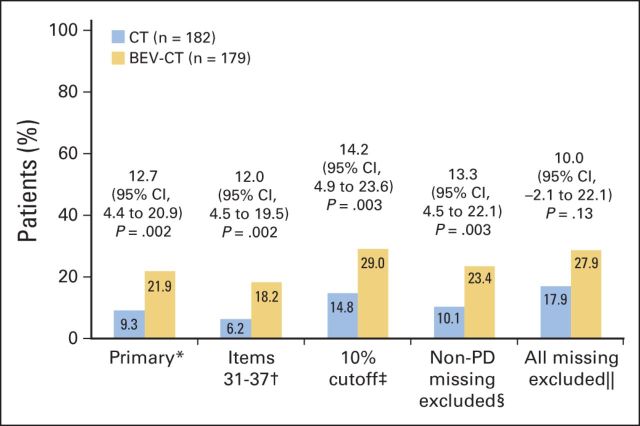
Fig 3.
Primary and sensitivity analyses of the primary hypothesis (≥ 15% improvement in abdominal/GI symptoms [European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Ovarian Cancer Module 28]). 95% Pearson-Clopper CIs with the Hauck-Anderson continuity correction for the difference between arms. (*) 15% cutoff, items 31 to 36, missing questionnaires counted as no improvement. (†) 15% cutoff, items 31 to 37, missing questionnaires counted as no improvement. (‡) 10% cutoff, items 31 to 36, missing questionnaires counted as no improvement. (§) 15% cutoff, items 31 to 36, questionnaires missing for reasons other than disease progression (PD) were not included in denominator. (‖) 15% cutoff, items 31 to 36, all missing questionnaires excluded from denominator. BEV, bevacizumab; CT, chemotherapy.